Review Article | Open Access
Chlorogenic acid regulates macrophages to improve inflammatory diseases
Ahmad Karim
1Department of Pathology, Virginia Commonwealth University Medical Center, Box 980662 1101 E, Marshall Street Richmond, VA 23298-0662, Virginia, USA.
Correspondence: Ahmad Karim (Department of Pathology, Virginia Commonwealth University Medical Center, Box 980662 1101 E, Marshall Street Richmond, VA 23298-0662, Virginia, USA; Email: Ammy.scie373@outlook.com).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2025, 5: 65-73. https://doi.org/10.32948/ajpt.2025.11.15
Received: 05 Aug 2025 | Accepted: 23 Sep 2025 | Published online: 13 Nov 2025
Key words chlorogenic acid, macrophages, inflammatory diseases, anti-inflammatory effects, oxidative stress, rheumatoid arthritis, inflammatory bowel disease, atherosclerosis
Macrophages have high plasticity, and environmental cues within the microenvironment guide the differentiation of these cells into pro-inflammatory M1 or anti-inflammatory M2 subtypes [6]. While M2 macrophages suppress inflammation and encourage tissue repair by secreting anti-inflammatory cytokines like IL-10, TGF-β, and tissue repair factors, M1 macrophages increase inflammation by releasing pro-inflammatory cytokines like TNF-α, IL-6, IL-1β, and promoting reactive oxygen species (ROS) [6, 7]. Therefore, regulating the polarization of macrophages is considered an important strategy for treating inflammatory diseases.
Drugs used for inflammatory diseases mainly include inflammatory cytokine antagonists, nonsteroidal anti-inflammatory drugs, immunosuppressants, and glucocorticoids [8]. However, these drugs have certain side effects, including susceptibility to drug resistance, gastrointestinal reactions, and liver and kidney damage [9]. Therefore, identifying effective therapeutics exhibiting negligible adverse events is an important direction in the ongoing advancements in inflammatory disorders. Over the past several years, natural compounds have received widespread attention based on their role in managing inflammatory disorders, low toxicity, and multi-target properties. With antioxidant, anti-inflammatory, and immunomodulatory properties, CGA is present in coffee, various teas, a spectrum of fruits, and numerous vegetables [10]. It can inhibit the NF-κB and MAPK signaling pathways [11, 12], regulate oxidative stress response, and regulate the polarization of macrophages to exert anti-inflammatory effects [10]. In LPS-induced RAW264.7 macrophages, studies have shown that CGA can dramatically reduce the expression of pro-inflammatory cytokines and encourage M2 macrophage polarization [13]. In addition, CGA has been explored to decrease the size of myocardial infarction and improve survival rate via lowering oxidative stress and inflammatory injury in rats [14].
Although CGA possesses therapeutic potential for inflammatory diseases, its precise mode of action and clinical use require more investigation. This review aims to discuss the recent progress in the regulation of macrophages by CGA to improve inflammatory diseases, and explore its future research directions and application prospects.
CGA, also known as coffee tannic acid, is found in the form of semi-hydrated yellow or white needle-like crystals which are transformed into an anhydrous state at 110°C. It can be heated with diluted hydrochloric acid to form caffeic acid. It belongs to polyphenol family, and is found in various plant species, but mainly in coffee, tea, apples, pears, and various vegetables. Chemically, it is composed of caffeic acid and quinic acid connected by ester bonds, with two aromatic ring structures and an unstable structure. Its solubility in water at room temperature (25 ℃) is about 4%. It is easily soluble in ethanol and acetone, slightly soluble in ethyl acetate, and difficult to dissolve in lipophilic organic solvents such as ether and chloroform [15]. CGA has several biological effects, including antioxidant, anti-inflammatory, and hypoglycemic impacts. The chemical structure of CGA endows it with unique biological activity, and the phenolic hydroxyl and ester bonds in its molecule give it strong antioxidant capacity, which can clear free radicals and inhibit oxidative stress reactions [16]. Its bioavailability in the human body in relatively high. After oral administration, it is absorbed into the intestine and distributed to various tissues across the entire body with multiple polyphenol-derived metabolites measured in human plasma [17].
Metabolism of CGA
CGA is a weak organic acid containing multiple hydroxyl groups. With limited membrane permeability, it exhibits relatively low oral absorption rate. In rats, the absolute bioavailability of orally administered CGA monomer at a dosage of 50 mg/kg is only 4.8% [18]. In humans, CGA is partially absorbed within the small bowel after oral ingestion. The absorption efficiency is potentially influenced by aspects including food categories and individual differences, accounting for about one-third of the total intake [19]. Although the absorption rate is not high, CGA and caffeic acid can still be effectively absorbed in the human body and enter systemic circulation.
In the small intestine, less than 1% of CGA is hydrolyzed by mucosal esterase, producing metabolites including caffeic, quinic, and ferulic acids. About two-thirds of CGA enters the cecum or colon, where the intestinal microbiome plays a significant role [19, 20]. The gut microbiota metabolizes CGA into caffeic acid, ferulic acid, quinic acid, hydroxycinnamic acid, and phenylpropanoid derivatives. There is a complex interaction between CGA and the gut microbiota. This interaction has been shown to affect the structure and function of the gut microbiota, thereby having a positive impact on various diseases. Wang Z.'s research shows that CGA reverses high-fat-diet-induced dysbiosis of the gut microbiota, including significant inhibition of the growth of Desulfoviridae, Ruminococcaceae, Molluscaceae, and Erysipelaceae, as well as increased growth of Bacteroidetes and Lactobacillaceae [21]. This indicates that CGA has potential application value in weight loss, prophylaxis, and pathophysiological modulation of metabolic disorders.
CGA and its metabolites absorbed into the bloodstream are distributed to various tissues and organs throughout the body through blood circulation. In the liver, these compounds undergo further metabolic reactions, predominantly mediated through the cytochrome P450 monooxygenase network, encompassing oxidative transformations, reductive processes, and hydrolytic cleavage. Phase II metabolism includes transformations that encompass glucuronidation and sulfation, which help increase the water solubility of compounds and facilitate their excretion from the body. CGA and its metabolites are mainly excreted from the body along with bile, and some can also be excreted through urine. Overall, CGA has a certain therapeutic effect on various systems in the body (Figure 1).
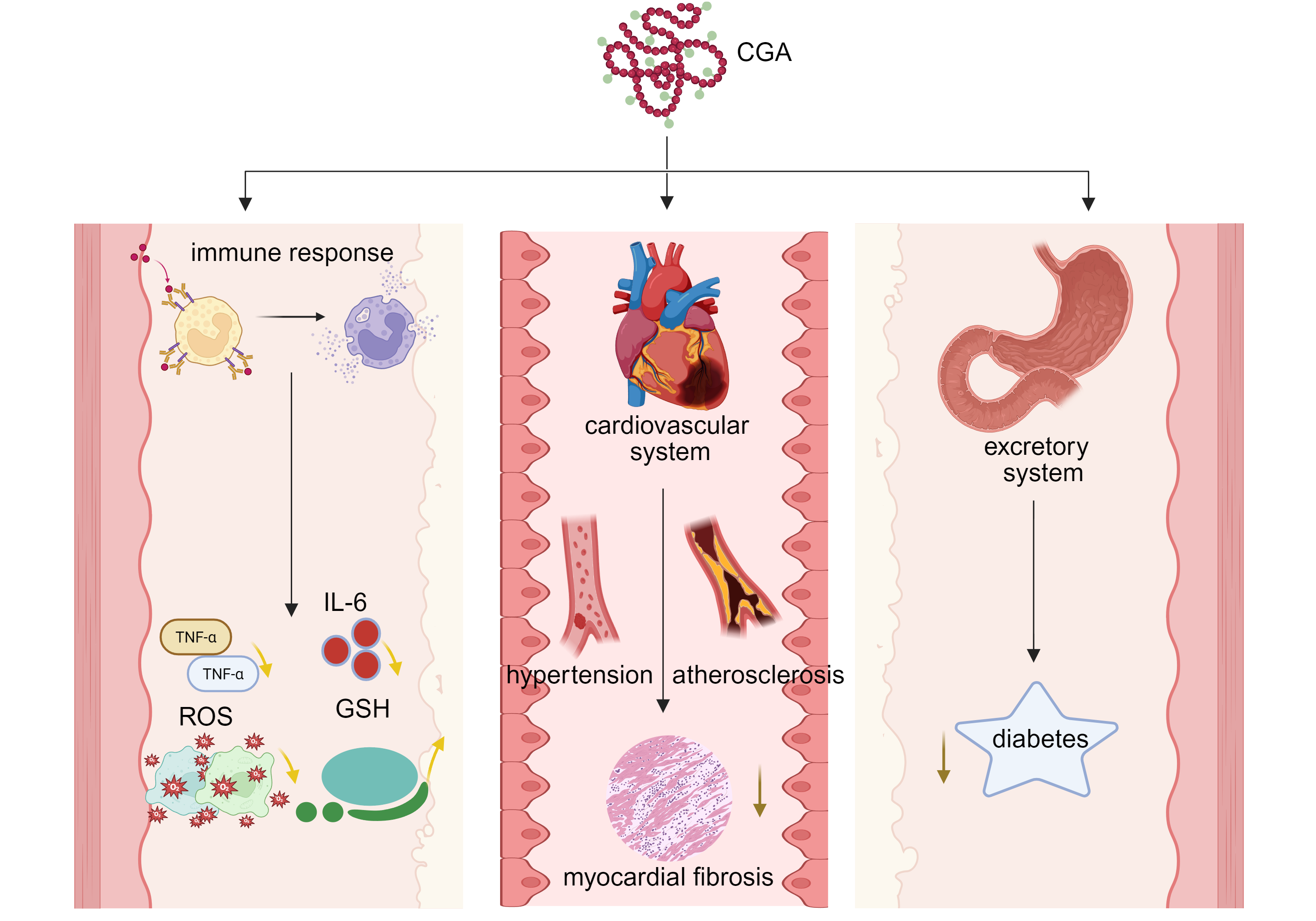 Figure 1. Effects of chlorogenic acid on immune response and systemic health. The multi-system effects of chlorogenic acid (CGA) on immune response and overall health were demonstrated. In the figure, CGA is the core and is expanded from three dimensions. Immune response module: CGA acts on immune cells, triggering the release of inflammatory mediators such as TNF - α and ROS, thereby regulating IL-6 levels and affecting the expression of antioxidant GSH. Cardiovascular system module: CGA participates in the process of blood pressure regulation (hypertension), vascular disease (atherosclerosis) and cardiac fibrosis (myocardial fibrosis). Excretion system module: CGA is associated with the occurrence and development of diabetes. CGA: chlorogenic acid; TNF-α: tumor necrosis factor-alpha; ROS: reactive oxygen species; IL-6: interleukin-6; GSH: glutathione.
Figure 1. Effects of chlorogenic acid on immune response and systemic health. The multi-system effects of chlorogenic acid (CGA) on immune response and overall health were demonstrated. In the figure, CGA is the core and is expanded from three dimensions. Immune response module: CGA acts on immune cells, triggering the release of inflammatory mediators such as TNF - α and ROS, thereby regulating IL-6 levels and affecting the expression of antioxidant GSH. Cardiovascular system module: CGA participates in the process of blood pressure regulation (hypertension), vascular disease (atherosclerosis) and cardiac fibrosis (myocardial fibrosis). Excretion system module: CGA is associated with the occurrence and development of diabetes. CGA: chlorogenic acid; TNF-α: tumor necrosis factor-alpha; ROS: reactive oxygen species; IL-6: interleukin-6; GSH: glutathione.
Acute lung injury
Infection by pathogenic microorganisms, weakened immunity, and other factors can lead to pneumonia. The accumulation of M1 macrophages and the generation of elevated concentrations of inflammation-related substances, forming an inflammatory cytokine storm, are considered the primary contributors to the progression of this condition. Lv B.'s study found that CGA improves LPS-elicited acute pulmonary injury by suppressing the lysine acetyltransferase 2A gene and suppressing the inflammatory cytokine storm [22]. They established a model of acute pulmonary injury through the administration of lipopolysaccharide into mice via the tail vein. The results showed that CGA inhibited inflammation and significantly improved respiratory function decline in mice resulting from the administration of lipopolysaccharide, through the inhibition of KAT2A expression [22]. Liu C. investigated the mechanism by which CGA attenuates lipopolysaccharide-induced acute pulmonary injury in murine models by modulating the miR-223/NLRP3 pathway. It was found that CGA can effectively alleviate lung injury and the inflammatory response [23].
The NLRP3 inflammasome constitutes a crucial element in the inflammatory reaction, and its activation leads to the maturation and release of IL-1β and IL-18 [24]. Chai X. reported that CGA prevents suppression of Lnc Neat1 gene expression, and NLRP3 inflammasome activation mitigates ischemia-reperfusion-induced myocardial damage and pyroptosis [25]. Although not directly applied in acute lung injury models, it also has certain reference value for acute lung injury. Subsequently, Xu Y. also discovered the characterization of NLRP3 inflammasome small molecule inhibitors and their potential use in acute lung injury [26].
Shi A. reported that an increase in Nrf2 activation was observed upon CGA treatment, and this enhanced transcription of Nrf2-associated antioxidant genes such as HO-1, NQO1, and GCLC [24]. Zeng J. studied the operational principle of CGA in combating Klebsiella pneumoniae-induced pneumonia in mouse experiments and found that CGA significantly inhibited the triggering of the NLRP3 inflammasome. The findings suggest that consuming CGA or foods rich in CGA in the diet improves Klebsiella pneumoniae-alleviated pneumonia, leading to the down-regulation of NLRP3 inflammasome activation [27].
In addition, Li QR infected mice with Klebsiella pneumoniae and treated them with CGA and a silencing information regulatory factor 1 (SIRT1) inhibitor (Selisistat). The results showed that CGA can regulate macrophage polarization in pneumonia, activate silent information regulator 1 (SIRT1), resulting in diminished acetylation and nuclear import of high mobility group protein B1 (HMGB1), which subsequently facilitates the acquisition of an anti-inflammatory phenotype in alveolar macrophages, and alleviates pneumonia [28]. Jain S.'s research demonstrated that CGA targets TLR4/3 to alleviate oxidative stress-induced NLRP3/NF-κB axis and improve acute respiratory distress syndrome. Studies have shown that CGA treatment normalized immune cell migration, reduced elevated levels of pro-inflammatory cytokines, and lowered the serum biomarker D-dimer for intravascular coagulation after CGA treatment [29]. He F. et al. showed that in vitro, CGA significantly suppresses LPS-induced inflammatory responses and enhances phagocytic activity in RAW264.7 cells. In vivo, CGA administration significantly alleviates lung inflammation and tissue damage, reduces lung bacterial load, promotes alveolar macrophage phagocytosis, improves the survival rate of CLP-induced ARDS mice, and significantly upregulates GPR37 expression both in vivo and in vitro [30]. CGA can enhance the phagocytosis of alveolar giant cells and improve acute respiratory distress syndrome through the activation of G-protein coupled receptor 37 (GPR37).
Inflammatory bowel disease
A collection of non-specific, long-term intestinal inflammatory illnesses, IBD mostly consists of Crohn's disease (CD) and ulcerative colitis (UC) [31, 32]. These two diseases have similar clinical symptoms, but there are differences in the location of onset, pathological features, and complications.
Continuous exposure to cadmium (Cd) is responsible for significant impairment of the hepatic and digestive systems. CGA has been demonstrated in earlier research to strengthen the intestinal barrier in weanling rats. Xue Y investigated the ameliorative influence of CGA, both in isolated form and within sunflower seed extract (SSE), on the growth dynamics, oxidative stress parameters, inflammation indicators, and gut barrier robustness in rats subjected to cadmium exposure [33]. The findings indicated that both CGA and SSE diminished Cd accumulation in the jejunum and augmented fecal Cd excretion. Combined treatment with CGA or SSE in rats was associated with a reduction in inflammation, an improvement in villous damage, a restoration of tight junction integrity, and a regain of body weight [33]. Zhang Z. demonstrated that CGA attenuated multiple effects of dextran sodium sulfate the symptoms of DSS-induced colitis included weight loss, a higher disease activity index and aggravated mucosal lesions by inhibiting the active NF-KB signaling pathway. Besides, CGA significantly inhibited the secretion of IFNγ, TNFα, and IL-6, and reduced the infiltration of CD3+ T cells, F4/80+ macrophages, and CD177+ neutrophils in the colon, which is caused by NF-κB pathway inhibition and an increased abundance of mucinophilic Akkermansia spp. contributing to the improvement of experimental colitis [34]. The MAPK signaling cascade is profoundly linked to the development of ulcerative colitis. Gao W. reported that CGA alleviates DSS-triggered ulcerative colitis in murine subjects substantially alleviating tissue inflammation and apoptotic events wherein the operative mechanism involves the MAPK/ERK/JNK signaling cascade [11].
Additionally, Wan F. demonstrated that CGA exerts antioxidant and anti-inflammatory effects and restores intestinal barrier function by activating the Nrf2/heme oxygenase-1 (HO-1) pathway, thereby effectively improving DSS-induced colitis. These results suggest that DSS-induced colitis can be improved through the attenuation of oxidative stress and inflammation, as well as the restoration of the intestinal barrier [35]. In summary, CGA regulates inflammatory and oxidative molecular mechanisms via multiple signaling pathways (Figure 2).
Rheumatoid arthritis (RA)
RA, a continuous and comprehensive autoimmune disease, typically attacks the synovium of the joints, resulting in joint damage that worsens over time, destruction and dysfunction, and may also cause systemic damage. It is characterized by joint swelling, pain, morning stiffness (lasting ≥1 hour), synovial hyperplasia, and formation of vascular cataracts, and gradual erosion of cartilage and bone, leading to joint deformity.
CGA is the main component of Caulis Lonicerae, an herbal medicine utilized in the therapy of RA. Lou, L showed that CGA had the capacity to block IL-6-mediated inhibition of inflammatory cell proliferation in RSC-364 cells by inducing programmed cell death, and suppressed the activation of the JAK/STAT and NF-κB signaling pathways in the inflammatory response through IL-6-mediated signaling, as well as the expression levels of important components in these signaling pathways, thereby inhibiting inflammatory proliferation of synovial cells. STAT and NF-κB molecular signaling routes were also affected, and the activation of these signaling pathways during the inflammatory response was prevented by blocking IL-6-mediated signaling, thereby inhibiting the inflammatory proliferation of synovial cells [36]. The current results suggest that CGA could be used as a new medicinal substance to inhibit synovial inflammatory proliferation by inducing synovial cell apoptosis in RA patients. Furthermore, Lou L. has revealed that CGA exhibits the potential to prevent IL-6-mediated signaling. It effectively suppressed the production of important molecules in the JAK/STAT and NF-κB signaling pathways, as well as inhibited the initiation of these signaling pathways involved in the inflammatory response via suppressing IL-6 signaling. It also induced apoptosis in RSC-364 cells, thereby limiting their expansion in response to inflammation [36]. Fu X. has reported that CGA can inhibit TNF-α-induced BAFF expression, and promote apoptosis in MH7A cells in a dose-dependent manner. It indicates that the presence of the NF-κB binding site in the BAFF promoter region is necessary for regulating this process [37]. The findings suggest that CGA could offer a new strategy for treating RA by focusing on BAFF as a key target of CGA.
Macrophages have been reported to be functionally heterogeneous in RA, as they can release both pro-inflammatory cytokines including TNF and IL-1β to encourage inflammatory reactions, and anti-inflammatory cytokines such as IL-10. Metabolic reprogramming of macrophages may be an important mechanism for the regulation of their functions [38]. Liu Q. synthesized calcium CGA nanoparticles (Ca-CGA NPs) by binding small molecules of CGA to Ca²⁺. Under the low pH conditions of lysosomes, the internalized Ca-CGA NPs were capable of dissolving to liberate free CGA and Ca²⁺. Findings from controlled in vitro tests showed that not only did Ca-CGA NPs improve BMSC osteogenic differentiation, but also promoted the phenotypic transition of macrophages from M1 to M2. In addition, in vivo tests verified that administering Ca-CGA NPs aided in the recuperation of a rat cranial bone defect model with osteogenic induction and immunomodulation [39]. In order to speed bone healing, a novel method based on Ca-CGA NPs was devised in this study to stimulate BMSC differentiation into osteoblasts and macrophage polarization into the M2 phenotype.
Atherosclerosis
Atherosclerosis, a prolonged inflammatory illness which is defined by the disruption of unstable atherosclerotic plaques, stenosis, or occlusion due to thrombosis and platelet aggregation, causes acute cardiovascular illness [40, 41]. Over the last few years, the role of CGA, a natural polyphenolic compound, in regulating the process of atherosclerosis (AS) has received increasing attention. In addition, caffeoylquinic acid (CQA) and feruloylquinic acid (FQA), two of its isomers which can raise antioxidant activity and dramatically lower total blood cholesterol, have shown promise. According to the docking results, the energy binding of CGA compounds and their isomers is less than that of the drug ibuprofen, which suggests that CGA compounds and their isomers are superior to the drug ibuprofen, and therefore they have anti-inflammatory potential and can be used to prevent atherosclerosis formation [42]. Meng C. found that CGA maintains cholesterol homeostasis by regulating NPC1L1 and HMGCR expression through PXR and SREBP2 signaling, along with their interaction with HSP90. This suggests that CGA has a potential role in regulating the atherosclerotic process [43]. Steinbauer S. found that components of elderberry (Sambucus nigra), including CGA, were able to prevent foam cell development without increasing hepatic lipogenesis by a high-content screen, a finding that supports the potential role of CGA in attenuating atherosclerotic lesions [44].
Atherosclerosis is a chronic inflammatory disease brought on by artery endothelial dysfunction, and macrophages are essential to the onset and course of the illness [45]. CGA significantly ameliorates the pathological process of atherosclerosis by modulating macrophage function. Francisco V showed that CGA can possess anti-inflammatory qualities via inhibiting the NF-κB pathway, cytokine production, and proteasomes, which supports the anti-inflammatory action of polyphenols in physiologically relevant cells [46]. CGA possesses antioxidant and anti-inflammatory effects and is capable of inhibiting the inflammatory response induced by atherosclerosis. Furthermore, little is known about the chemical processes that underlie the suppression of oxLDL-driven oxidative damage in human endothelial cells. Recently, according to data from Tsai, K., CGA pretreatment increased the level of SIRT1 deacetylase activity, reduced oxidative stress and mitochondrial biogenesis failure brought on by oxLDL, and corrected oxLDL-impaired SIRT1 and AMPK/PGC-1 activity [47]. This finding provides fresh insight into the potential molecular pathways by which CGA inhibits mitochondrial dysfunction and endothelial oxidative stress brought on by oxLDL by activating SIRT1 and modifying the AMPK/PGC-1 signaling pathway.
In addition to the use of CGA alone, there have been attempts to see if the combination would be more effective. In order to investigate whether CGA and caffeic acid (CA) could reduce the ability of macrophages to accumulate lipids, Marino, M. conducted a study. The results showed that the CGA + CA mixture reduced the ability to store lipids in macrophages, and further studies revealed that the reduction in lipid storage was mediated by diminished levels of the transcription factors C/EBPβ and PPAR-γ [48]. In addition, CGA has proven to be beneficial for the treatment of various other diseases, such as liver injury [49, 50], liver inflammation and fibrosis [51], obesity [52], healthy gut development [53], inflammatory damage [54] (Table 1).
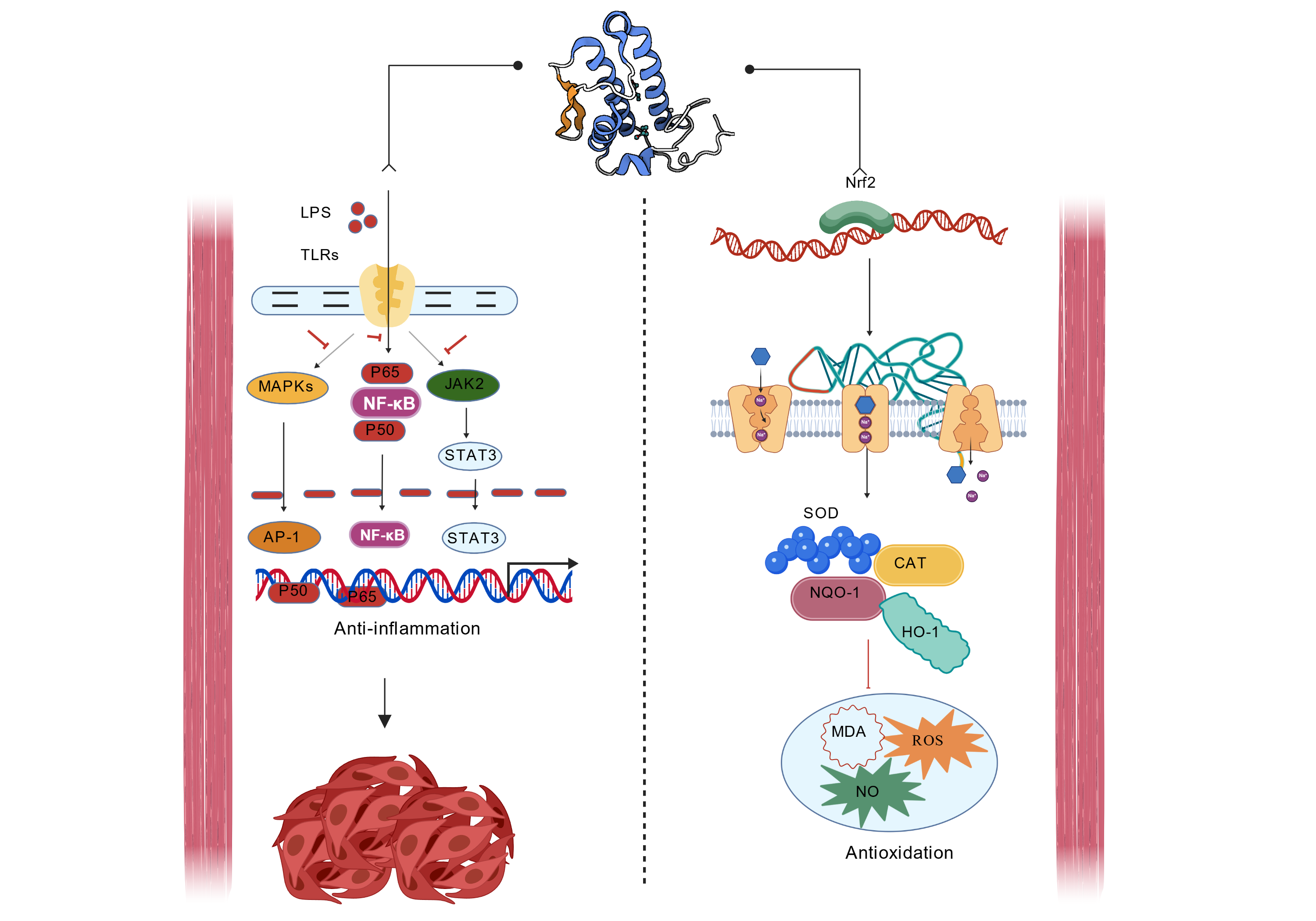 Figure 2. Molecular mechanisms of chlorogenic acid in anti-inflammation and antioxidation. Anti-inflammatory mechanism: LPS activate signaling pathways such as MAPKs, PI3K, JAK2 by binding to TLRs, thereby regulating transcription factors such as NF-κB, STAT3, AP-1 and ultimately inhibiting inflammatory responses; Antioxidant mechanism: After Nrf2 enters the nucleus, it initiates the expression of antioxidant enzymes such as SOD, CAT, NQO-1, HO-1, etc., which help clear oxidative products such as MDA, ROS and NO and exerting antioxidant effects. LPS: lipopolysaccharide; TLRs: toll-like receptors; MAPKs: mitogen-activated protein kinases; PI3K: phosphoinositide 3-kinase; JAK2: janus kinase 2; NF-κB: nuclear-factor kappa-B; STAT3: signal transducer and activator of transcription 3; AP-1: activator protein-1; Nrf2: nuclear factor erythroid 2-related factor 2; SOD: superoxide dismutase; CAT: catalase; NQO-1: NAD(P)H quinone dehydrogenase 1; HO-1: heme oxygenase-1; MDA: malondialdehyde; ROS: reactive oxygen species; NO: nitric oxide.
Figure 2. Molecular mechanisms of chlorogenic acid in anti-inflammation and antioxidation. Anti-inflammatory mechanism: LPS activate signaling pathways such as MAPKs, PI3K, JAK2 by binding to TLRs, thereby regulating transcription factors such as NF-κB, STAT3, AP-1 and ultimately inhibiting inflammatory responses; Antioxidant mechanism: After Nrf2 enters the nucleus, it initiates the expression of antioxidant enzymes such as SOD, CAT, NQO-1, HO-1, etc., which help clear oxidative products such as MDA, ROS and NO and exerting antioxidant effects. LPS: lipopolysaccharide; TLRs: toll-like receptors; MAPKs: mitogen-activated protein kinases; PI3K: phosphoinositide 3-kinase; JAK2: janus kinase 2; NF-κB: nuclear-factor kappa-B; STAT3: signal transducer and activator of transcription 3; AP-1: activator protein-1; Nrf2: nuclear factor erythroid 2-related factor 2; SOD: superoxide dismutase; CAT: catalase; NQO-1: NAD(P)H quinone dehydrogenase 1; HO-1: heme oxygenase-1; MDA: malondialdehyde; ROS: reactive oxygen species; NO: nitric oxide.
|
Table 1. Research on the application of chlorogenic acid in diseases. |
|||
|
Disease type |
Animal/Cell models |
Result |
References |
|
Liver injury |
Mice |
CGA counteracts AP-induced liver injury at different levels by preventing cell apoptosis and oxidative stress damage; The GSH and Trx antioxidant systems, as well as the mitogen activated MAPK signaling cascade, are involved in the protective mechanism. |
[49] |
|
Liver inflammation and fibrosis |
Rat |
Inhibition of CCl4-induced rat liver fibrosis may have a protective effect due to inhibition of the TLR4/MyD88/NF-κB signaling pathway. |
[51] |
|
Obesity |
3T3-L1 cells |
The main colonic metabolite of CGA can counteract TNF α- induced inflammation and oxidative stress in 3T3-L1 cell lines, and has the potential to combat obesity. |
[52] |
|
Healthy development of intestines |
Weaned piglets |
CGA improves intestinal development by inhibiting mucosal inflammation and cell apoptosis in weaned piglets. |
[53] |
|
Inflammatory injury |
RAW264.7 macrophages |
By regulating CD36/AMPK/PGC-1 α to alleviate oxidative stress, it can contribute to the development of inflammatory diseases. |
[54] |
|
Acute liver injury |
Mice |
CGA affects the kinase activity of IRAK4, inhibits the autophosphorylation of IRAK4 stimulated by various TLR agonists, IL-1 α, or high mobility box-1 in peritoneal macrophages of C57BL/6 or C3H/HeJ mice, and enhances the transcriptional activity of NF-κB or AP-1. |
[50] |
(1) CGA has low oral bioavailability, a short retention time in the body, and needs repeated administration. If injected intravenously, it will lead to excessive local CGA, and excessive CGA may bind to protein groups and cause allergic adverse reactions [55].
(2) Caffeic acid and ferulic acid, which are metabolites of CGA, also exhibit anti-inflammatory activity, and their mechanisms for exerting CGA activity need to be further investigated [56].
(3) The primary active component of many traditional Chinese medications is CGA, but the effects of other ingredients in traditional Chinese medicines and compound formulations on their pharmacokinetics are unknown [57].
An early stage of cirrhosis that affects many people is liver fibrosis, posing a serious health risk for which there is no viable treatment. The polyphenol monomer CGA was delivered to the wounded liver for the first time in this study using tetrahedral framework nucleic acid nanoparticles as carriers. Through this delivery approach, CGA's bioavailability and stability were enhanced, laying the foundation for its antifibrotic and antioxidant effects [58]. In addition, studies have shown that nanogels are promising drug delivery frameworks for targeting collagen protein-induced arthritis, utilizing chitosan and interconnections used to encapsulate CGAs [59]. Zhou H. made phospholipid-based in situ gels with CGA-PG. In vitro and in vivo, CGA-PG demonstrated appropriate slow-release behavior, high drug loading capacity, good fluidity, and easy injectability. The results demonstrated that CGA-PG could stop tumor growth without causing any serious side effects [60]. All things considered, CGA-PG might be a potential long-term medication administration method with superior therapeutic outcomes for gliomas and hepatocellular cancer, whether it will again continue to be modified for applications in inflammatory diseases remains to be seen. Encapsulation of CGA utilizing a self-microemulsifying drug delivery approach increased its oral bioavailability and promoted drug accumulation in the mesenteric lymph nodes to achieve amelioration of inflammation [61]. Specific targeting of nanoparticles for colonic inflammation is controlled by loading CGA using combined ultrasonic emulsification. The metabolites of CGA, including caffeic acid and ferulic acid, also possess anti-inflammatory properties. This is followed by degradation of pectin by colonic flora and pectinase generated by a magnetic field applied to the colon, and this oral colonic nano-delivery system could have a promising future as a new therapeutic modality [62].
In addition, the low oral bioavailability of CGA and the anti-inflammatory function of its metabolites are among the bottlenecks limiting its clinical application. How to improve the bioavailability of CGA, extend its half-life in vivo, and reduce the side effects through drug delivery systems are key tasks for future research. Nanotechnology and targeted drug delivery systems may provide new solutions to enhance the efficacy of CGA.
CGA, as a natural polyphenolic compound, has been preliminarily demonstrated to have potential application in a variety of inflammatory diseases. Future clinical studies are necessary to validate the effectiveness and safety of CGA through multicenter, large-sample randomized controlled trials, with the aim of providing a new therapeutic option associated with mild side effects for the management of inflammatory diseases.
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
The entire article was drafted and revised by Ahmed Karim, who also created the tables and charts and submitted the final version.
Competing interests
The authors declare no competing interests.
- Haftcheshmeh SM, Abedi M, Mashayekhi K, Mousavi MJ, Navashenaq JG, Mohammadi A, Momtazi-Borojeni AA: Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother Res 2022, 36(3): 1216-1230.
- Wang M, Pan H, Zhai Y, Li H, Huang L, Xie Z, Wen C, Li X: Bidirectional association between rheumatoid arthritis and chronic obstructive pulmonary disease: a systematic review and meta-analysis. Front Immunol 2024, 15: 1494003.
- Barnes PJ: Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016, 138(1): 16-27.
- Feng W, Chen G, Cai D, Zhao S, Cheng J, Shen H: Inflammatory Bowel Disease and Risk of Ischemic Heart Disease: An Updated Meta-Analysis of Cohort Studies. J Am Heart Assoc 2017, 6(8): e005892.
- Guo W, Li Z, Anagnostopoulos G, Kong WT, Zhang S, Chakarov S, Shin A, Qian J, Zhu Y, Bai W et al: Notch signaling regulates macrophage-mediated inflammation in metabolic dysfunction-associated steatotic liver disease. Immunity 2024, 57(10): 2310-2327.e6.
- Peng Y, Zhou M, Yang H, Qu R, Qiu Y, Hao J, Bi H, Guo D: Regulatory Mechanism of M1/M2 Macrophage Polarization in the Development of Autoimmune Diseases. Mediators Inflamm 2023, 2023: 8821610.
- Yunna C, Mengru H, Lei W, Weidong C: Macrophage M1/M2 polarization. Eur J Pharmacol 2020, 877: 173090.
- McInnes IB, Gravallese EM: Immune-mediated inflammatory disease therapeutics: past, present and future. Nat Rev Immunol 2021, 21(10): 680-686.
- El Agaty SM, Khedr S, Mostafa DKM, Wanis NA, Abou-Bakr DA: Protective role of melatonin against diclofenac-induced acute kidney injury. Life Sci 2024, 353: 122936.
- Miao M, Xiang L: Pharmacological action and potential targets of chlorogenic acid. Adv Pharmacol 2020, 87: 71-88.
- Gao W, Wang C, Yu L, Sheng T, Wu Z, Wang X, Zhang D, Lin Y, Gong Y: Chlorogenic Acid Attenuates Dextran Sodium Sulfate-Induced Ulcerative Colitis in Mice through MAPK/ERK/JNK Pathway. Biomed Res Int 2019, 2019: 6769789.
- Nguyen V, Taine EG, Meng D, Cui T, Tan W: Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16(7): 924.
- Linghu KG, Ma QS, Zhao GD, Xiong W, Lin L, Zhang QW, Bian Z, Wang Y, Yu H: Leocarpinolide B attenuates LPS-induced inflammation on RAW264.7 macrophages by mediating NF-κB and Nrf2 pathways. Eur J Pharmacol 2020, 868: 172854.
- Wang D, Tian L, Lv H, Pang Z, Li D, Yao Z, Wang S: Chlorogenic acid prevents acute myocardial infarction in rats by reducing inflammatory damage and oxidative stress. Biomed Pharmacother 2020, 132: 110773.
- Gil M, Wianowska D: Chlorogenic acids – their properties, occurrence and analysis. Annales UMCS, Chemia 2017, 72(1): 61-104.
- Izu GO, Mfotie Njoya E, Tabakam GT, Nambooze J, Otukile KP, Tsoeu SE, Fasiku VO, Adegoke AM, Erukainure OL, Mashele SS et al: Unravelling the Influence of Chlorogenic Acid on the Antioxidant Phytochemistry of Avocado (Persea americana Mill.) Fruit Peel. Antioxidants (Basel) 2024, 13(4): 456.
- Abourashed EA: Bioavailability of Plant-Derived Antioxidants. Antioxidants 2013, 2(4): 309-325.
- Stalmach A, Steiling H, Williamson G, Crozier A: Bioavailability of chlorogenic acids following acute ingestion of coffee by humans with an ileostomy. Arch Biochem Biophys 2010, 501(1): 98-105.
- Zhou W, Wang H, Zhu X, Shan J, Yin A, Cai B, Di L: Improvement of Intestinal Absorption of Forsythoside A and Chlorogenic Acid by Different Carboxymethyl Chitosan and Chito-oligosaccharide, Application to Flos Lonicerae - Fructus Forsythiae Herb Couple Preparations. Plos One 2013, 8(5): e63348.
- lin BI, yao LI, Duqiu, qing DI: Study on O/W Partition Coefficient of Chlorogenic Acid and Rats Intestinal Absorption Kinetics. J Nanjing Univers Tradition Chin Med 2013, 29(6): 572-575.
- Wang Z, Lam KL, Hu J, Ge S, Zhou A, Zheng B, Zeng S, Lin S: Chlorogenic acid alleviates obesity and modulates gut microbiota in high-fat-fed mice. Food Sci Nutr 2019, 7(2): 579-588.
- Lv B, Guo J, Du Y, Chen Y, Zhao X, Yu B, Liu J, Cui T, Mao H, Wang X, Gao X: Chlorogenic acid reduces inflammation by inhibiting the elevated expression of KAT2A to ameliorate lipopolysaccharide-induced acute lung injury. Br J Pharmacol 2023, 180(16): 2156-2171.
- Liu C, Cheng X, Sun J, Zhang S, Zhang Q: Mechanism of chlorogenic acid reducing lipopolysaccharide-induced acute lung injury in mice by regulating miR-223/NLRP3 axis. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2022, 47(3): 280-288.
- Shi A, Shi H, Wang Y, Liu X, Cheng Y, Li H, Zhao H, Wang S, Dong L: Activation of Nrf2 pathway and inhibition of NLRP3 inflammasome activation contribute to the protective effect of chlorogenic acid on acute liver injury. Int Immunopharmacol 2018, 54: 125-130.
- Chai X, Liang Z, Zhang J, Ding J, Zhang Q, Lv S, Deng Y, Zhang R, Lu D: Chlorogenic acid protects against myocardial ischemia-reperfusion injury in mice by inhibiting Lnc Neat1/NLRP3 inflammasome-mediated pyroptosis. Sci Rep 2023, 13(1): 17803.
- Xu Y, Biby S, Guo C, Liu Z, Cai J, Wang XY, Zhang S: Characterization of a small molecule inhibitor of the NLRP3 inflammasome and its potential use for acute lung injury. Bioorg Chem 2024, 150: 107562.
- Zeng J, Wan X, Liu T, Xiong Y, Xiang G, Peng Y, Zhu R, Zhou Y, Liu C: Chlorogenic acid ameliorates Klebsiella pneumoniae-induced pneumonia in immunosuppressed mice via inhibiting the activation of NLRP3 inflammasomes. Food Funct 2021, 12(19): 9466-9475.
- Li QR, Tan SR, Yang L, He W, Chen L, Shen FX, Wang Z, Wang HF: Mechanism of chlorogenic acid in alveolar macrophage polarization in Klebsiella pneumoniae-induced pneumonia. J Leukoc Biol 2022, 112(1): 9-21.
- Jain S, Saha P, Syamprasad NP, Panda SR, Rajdev B, Jannu AK, Sharma P, Naidu VGM: Targeting TLR4/3 using chlorogenic acid ameliorates LPS+POLY I:C-induced acute respiratory distress syndrome via alleviating oxidative stress-mediated NLRP3/NF-κB axis. Clin Sci (Lond) 2023, 137(10): 785-805.
- He F, Gao F, Cai N, Jiang M, Wu C: Chlorogenic acid enhances alveolar macrophages phagocytosis in acute respiratory distress syndrome by activating G protein-coupled receptor 37 (GPR 37). Phytomedicine 2022, 107: 154474.
- Nabatov AA: The vesicle-associated function of NOD2 as a link between Crohn's disease and mycobacterial infection. Gut Pathog 2015, 7(1): 1.
- Szilagyi A, Xue X: Comparison of geographic distributions of Irritable Bowel Syndrome with Inflammatory Bowel Disease fail to support common evolutionary roots: Irritable Bowel Syndrome and Inflammatory Bowel Diseases are not related by evolution. Med Hypotheses 2018, 110: 31-37.
- Xue Y, Huang F, Tang R, Fan Q, Zhang B, Xu Z, Sun X, Ruan Z: Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague-Dawley rats. Food Chem Toxicol 2019, 133: 110751.
- Zhang Z, Wu X, Cao S, Cromie M, Shen Y, Feng Y, Yang H, Li L: Chlorogenic Acid Ameliorates Experimental Colitis by Promoting Growth of Akkermansia in Mice. Nutrients 2017, 9(7): 677.
- Wan F, Cai X, Wang M, Chen L, Zhong R, Liu L, Yi B, Hou F, Zhang H: Chlorogenic acid supplementation alleviates dextran sulfate sodium (DSS)-induced colitis via inhibiting inflammatory responses and oxidative stress, improving gut barrier integrity and Nrf-2/HO-1 pathway. J Functional Foods 2021, 87: 104808.
- Lou L, Zhou J, Liu Y, Wei Y, Zhao J, Deng J, Dong B, Zhu L, Wu A, Yang Y, Chai L: Chlorogenic acid induces apoptosis to inhibit inflammatory proliferation of IL-6-induced fibroblast-like synoviocytes through modulating the activation of JAK/STAT and NF-κB signaling pathways. Exp Ther Med 2016, 11 (5): 2054-2060.
- Fu X, Lyu X, Liu H, Zhong D, Xu Z, He F, Huang G: Chlorogenic Acid Inhibits BAFF Expression in Collagen-Induced Arthritis and Human Synoviocyte MH7A Cells by Modulating the Activation of the NF-κB Signaling Pathway. J Immunol Res 2019, 2019: 8042097.
- Siouti E, Andreakos E: The many facets of macrophages in rheumatoid arthritis. Biochem Pharmacol 2019, 165: 152-169.
- Liu Q, Zhang S, Shi L, Shi J, Sun C, Wang J, Zhou W, Zhou H, Shan F, Wang H et al: Osteogenic Induction and Anti-Inflammatory Effects of Calcium-Chlorogenic Acid Nanoparticles Remodel the Osteoimmunology Microenvironment for Accelerating Bone Repair. Adv Healthc Mater 2024, 13(29): e2401114.
- Pedro-Botet J, Climent E, Benaiges D: Atherosclerosis and inflammation. New therapeutic approaches. Med Clin (Barc) 2020, 155(6): 256-262.
- Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R: Research Progress on the Relationship between Atherosclerosis and Inflammation. Biomolecules 2018, 8(3): 80.
- Nurarifa R, Rachmawati DE, Utari D: Molecular Docking Chlorogenic Acid and Isomer Compound as Cyclooxygenase-2 (COX-2) Inhibitor in Atherosclerosis. JSMAR Tech 2020, 2(1): 22-27.
- Meng C, Zhou L, Huang L, Gu Q, Du X, Wang C, Liu F, Xia C: Chlorogenic acid regulates the expression of NPC1L1 and HMGCR through PXR and SREBP2 signaling pathways and their interactions with HSP90 to maintain cholesterol homeostasis. Phytomedicine 2024, 123: 155271.
- Steinbauer S, König A, Neuhauser C, Schwarzinger B, Stangl H, Iken M, Weghuber J, Röhrl C: Elder (Sambucus nigra), identified by high-content screening, counteracts foam cell formation without promoting hepatic lipogenesis. Sci Rep 2024, 14(1): 3547.
- Dhawan UK, Singhal A, Subramanian M: Dead cell and debris clearance in the atherosclerotic plaque: Mechanisms and therapeutic opportunities to promote inflammation resolution. Pharmacol Res 2021, 170: 105699.
- Francisco V, Costa G, Figueirinha A, Marques C, Pereira P, Miguel Neves B, Celeste Lopes M, García-Rodríguez C, Teresa Cruz M, Teresa Batista M: Anti-inflammatory activity of Cymbopogon citratus leaves infusion via proteasome and nuclear factor-κB pathway inhibition: contribution of chlorogenic acid. J Ethnopharmacol 2013, 148(1): 126-134.
- Tsai K-L, Hung C, Chan S-H, Hsieh P-L, Ou H, Cheng Y-H, Chu PM: Chlorogenic Acid Protects Against oxLDL‐Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol Nutr Food Res 2018, 62(11): e1700928.
- Marino M, Del Bo′ C, Tucci M, Venturi S, Mantegazza G, Taverniti V, Møller P, Riso P, Porrini M: A mix of chlorogenic and caffeic acid reduces C/EBPß and PPAR-γ1 levels and counteracts lipid accumulation in macrophages. Eur J Nutr 2022,61(2): 1003-1004.
- Ji L, Jiang P, Lu B, Sheng Y, Wang X, Wang Z: Chlorogenic acid, a dietary polyphenol, protects acetaminophen-induced liver injury and its mechanism. J Nutr Biochem 2013, 24(11): 1911-1919.
- Park SH, Baek SI, Yun J, Lee S, Yoon DY, Jung JK, Jung SH, Hwang BY, Hong JT, Han SB, Kim Y: IRAK4 as a molecular target in the amelioration of innate immunity-related endotoxic shock and acute liver injury by chlorogenic acid. J Immunol 2015, 194(3): 1122-1130.
- Shi H, Dong L, Jiang J, Zhao J, Zhao G, Dang X, Lu X, Jia M: Chlorogenic acid reduces liver inflammation and fibrosis through inhibition of toll-like receptor 4 signaling pathway. Toxicology 2013, 303: 107-114.
- Goya L, Sánchez-Medina A, Redondo-Puente M, Dupak R, Bravo L, Sarriá B: Main Colonic Metabolites from Coffee Chlorogenic Acid May Counteract Tumor Necrosis Factor-α-Induced Inflammation and Oxidative Stress in 3T3-L1 Cells. Molecules 2023, 29(1): 88.
- Chen J, Xie H, Chen D, Yu B, Mao X, Zheng P, Yu J, Luo Y, Luo J, He J: Chlorogenic Acid Improves Intestinal Development via Suppressing Mucosa Inflammation and Cell Apoptosis in Weaned Pigs. ACS Omega 2018, 3(2): 2211-2219.
- Gu T, Zhang Z, Liu J, Chen L, Tian Y, Xu W, Zeng T, Wu W, Lu L: Chlorogenic Acid Alleviates LPS-Induced Inflammation and Oxidative Stress by Modulating CD36/AMPK/PGC-1α in RAW264.7 Macrophages. Int J Mol Sci 2023, 24(17): 13516.
- Zhang JJ, Luo QS, Li QQ, Xu Q, Geng X, Xiong JH: Fabrication and characterization of TPGS-modified chlorogenic acid liposomes and its bioavailability in rats. RSC Adv 2024, 14(35): 25289-25300.
- Huang J, Xie M, He L, Song X, Cao T: Chlorogenic acid: a review on its mechanisms of anti-inflammation, disease treatment, and related delivery systems. Front Pharmacol 2023, 14: 1218015.
- Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, Ahmad F, Babazadeh D, FangFang X, Modarresi-Ghazani F et al: Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed Pharmacother 2018, 97: 67-74.
- Yao L, Li J, Qin X, Liu Z, Jiang Y, Zhang T, Lin Y: Antifibrotic and antioxidant effects of a tetrahedral framework nucleic acid-based chlorogenic acid delivery system. ACS Materials Letters 2023, 5(4): 1153-1163.
- Ma Y, Song Y, Ma F, Chen G: A Potential Polymeric Nanogel System for Effective Delivery of Chlorogenic Acid to Target Collagen-Induced Arthritis. J Inorg Organomet Polym Mater 2020, 30(7): 2356-2365.
- Zhou H, Chen D, Gong T, He Q, Guo C, Zhang P, Song X, Ruan J, Gong T: Chlorogenic acid sustained-release gel for treatment of glioma and hepatocellular carcinoma. Eur J Pharm Biopharm 2021, 166: 103-110.
- Ye J, Gao Y, Ji M, Yang Y, Wang Z, Wang B, Jin J, Li L, Wang H, Xu X et al: Oral SMEDDS promotes lymphatic transport and mesenteric lymph nodes target of chlorogenic acid for effective T-cell antitumor immunity. J Immunother Cancer 2021, 9(7): e002753.
- Zhu H, Zhang L, Kou F, Zhao J, Lei J, He J: Targeted therapeutic effects of oral magnetically driven pectin nanoparticles containing chlorogenic acid on colon cancer. Particuology 2024, 84: 53-59.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript