Review Article | Open Access
Pharmacokinetics and pharmacodynamics of Tarlatamab, DLL3-Targeted FDA-approved Bispecific T-Cell Engager
Ahmed Attia Ahmed Abdelmoaty1, Ahmed Gamal Badran1
1Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt.
Correspondence: Ahmed Attia Ahmed Abdelmoaty (Department of Pharmacology, Faculty of Pharmacy, Zagazig University, Zagazig 44519, Egypt; E-mail: abdelmoaty14@lzu.edu.cn).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2025, 5: 44-52. https://doi.org/10.32948/ajpt.2025.04.15
Received: 05 May 2025 | Accepted: 23 Aug 2025 | Published online: 28 Sep 2025
Small-cell lung cancer (SCLC) is a common aggressive cancer type, that exhibits overall lower rate of survival and poor prognosis. Treatment options of SCLC are limited including chemotherapy, radiotherapy, and surgery to dissect tumors. However, these therapies are not very effective in treating SCLCs, and no available therapies are in third-line or beyond. The notch signaling pathway plays a central role in regulating cell proliferation, survival, and maintenance. Notch signaling in SCLC is dysregulated and induces oncogenicity. DLL3 is a notch ligand whose expression is nominal in normal conditions and the DLL3 is overexpressed in SCLC which promotes tumor cell proliferation, migration, and invasiveness. Over 85% of human SCLC express elevated DLL3 on the cell surface. Therefore, targeting DLL3 is a promising therapeutic approach to treat SCLC. Bispecific T-cell engagers (BiTEs) molecule binds to DLL3 and CD3 simultaneously leading to T-cell activation and T-cell-induced tumor eradication. Tarlatamab is a half-life extended DLL3-targeted T-cell-engaging bispecific antibody (BsAb) that exhibited superior antitumor efficacy in the preclinical in vitro and in vivo model. Tarlatamab is the only DLL3-engaging BiTE molecule that was approved by the USFDA on May 16th, 2024 under the brand name Imdelltra (Amgen Inc). It showed higher clinical efficacy and the pharmacodynamic study reported that higher T-cell activation and IFN-γ elevation were mediated after the first dose of tarlatamab. Manageable safety profile with higher efficacy rate was reported in the clinical study. In this review, we present immune therapy, and pharmacokinetic and pharmacodynamic profiles of tarlatamab based on the clinical study reports.
Key words tarlatamab, BiTE, pharmacokinetic, pharmacodynamic, clinical study, T-cell
A key factor limiting the efficacy of immune checkpoint blockade in most SCLC patients may be tumor-mediated downregulation of Major Histocompatibility Complex class I (MHC-I), which is essential for antigen presentation to CD8⁺ cytotoxic T lymphocytes [10, 11]. While epigenetic approaches to restore MHC-I expression are under investigation, an alternative therapeutic strategy involves circumventing conventional antigen presentation pathways entirely using bispecific T-cell engagers (BiTEs). These engineered antibodies simultaneously bind tumor-specific surface antigens and CD3 on T cells, forcibly inducing immunological synapse formation, T-cell activation, and subsequent tumor cell lysis [12-16].
Several anticancer monoclonal (mAbs) and bispecific antibodies (BsAbs) were approved by FDA in 2024 (Table 1). Tarlatamab is one of those BsAbs for solid tumors which has higher clinical efficacy. Tarlatamab is a first-in-class BiTE targeting delta-like ligand 3 (DLL3), a Notch pathway ligand overexpressed in SCLC, and the CD3ε subunit on T cells (Figure 1). Preclinical studies demonstrate that tarlatamab induces potent T-cell-mediated cytotoxicity against DLL3⁺ SCLC cells in vitro and drives significant tumor regression in disseminated orthotopic SCLC models in vivo [17, 18]. As the inaugural DLL3-directed immunotherapeutic agent to enter clinical trials, tarlatamab represents a paradigm-shifting approach for overcoming SCLC’s immune-evasion mechanisms. Tarlatamab, the first and exclusively DLL3-targeting bispecific T-cell engager (BiTE), elicits an immunotherapeutic response by directing the patient’s immune system against DLL3-expressing neoplastic cells. The molecule operates via dual binding to CD3 on T lymphocytes and DLL3 on tumor cells, the latter being a surface protein overexpressed in 85–96% of small cell lung carcinoma (SCLC) cases while exhibiting minimal expression in normal tissues. Upon simultaneous engagement of both receptors, T-cell activation ensues, culminating in the formation of cytolytic synapses that mediate tumor cell lysis, thereby demonstrating potent oncolytic efficacy. In this review, we present the overview of tarlatamab [19-24]. Herein, we highlight the therapeutic response, efficacy, pharmacokinetic and pharmacodynamic analysis, mechanism of action, adverse effect, and ADME of Tarlatamab.
|
Table 1. Anti-tumor mAbs and BsAb approved by FDA in 2024. |
||||||
|
Drug Name (Brand) |
Target |
Type |
Clinical efficacy data |
Approved Indication |
Manufacturer |
Year |
|
Epcoritamab (Epkinly)[25-27] |
CD3 × CD20 |
Bispecific |
ORR: 61% (CR: 38%) in R/R DLBCL (EPCORE NHL-1) |
R/R DLBCL, Follicular Lymphoma |
Genmab/AbbVie |
2024 |
|
Tarlatamab (Imdelltra)[28] |
DLL3 × CD3 |
Bispecific |
ORR: 40% (mDoR: 9.7 mos) in SCLC (DeLLphi-301) |
SCLC (2L+) |
Amgen |
2024 |
|
Elranatamab (Elrexfio)[29-31] |
BCMA × CD3 |
Bispecific |
ORR: 58% (≥VGPR: 33%) in R/R MM (MagnetisMM-3) |
R/R Multiple Myeloma |
Pfizer |
2024 |
|
Amivantamab (Rybrevant)[32, 33] |
EGFR × c-MET |
Bispecific |
ORR: 37% (mPFS: 6.7 mos) in EGFR Exon20+ NSCLC (CHRYSALIS-2) |
EGFR Exon20+ NSCLC |
J&J |
2024 |
|
Datopotamab deruxtecan (Dato-DXd)[34-37] |
TROP2 (ADC) |
ADC |
PFS: 6.9 vs 4.9 mos (vs chemo) in HR+/HER2- BC (TROPION-Breast01) |
HR+/HER2- BC, NSCLC |
Daiichi Sankyo/AstraZeneca |
2024 |
|
Zolbetuximab (Vyloy)[38-41] |
Claudin 18.2 |
mAb |
mPFS: 8.2 vs 6.8 mos (vs placebo + chemo) in Gastric/GEJ (SPOTLIGHT) |
CLDN18.2+ Gastric/GEJ |
Astellas Pharma |
2024 |
|
Pemigatinib (Pemazyre) + mAb[42-45] |
FGFR1-3 |
TKI + mAb |
ORR: 37% (mDoR: 8.1 mos) in FGFR2+ Cholangiocarcinoma (FIGHT-302) |
Cholangiocarcinoma |
Incyte |
2024 (combo) |
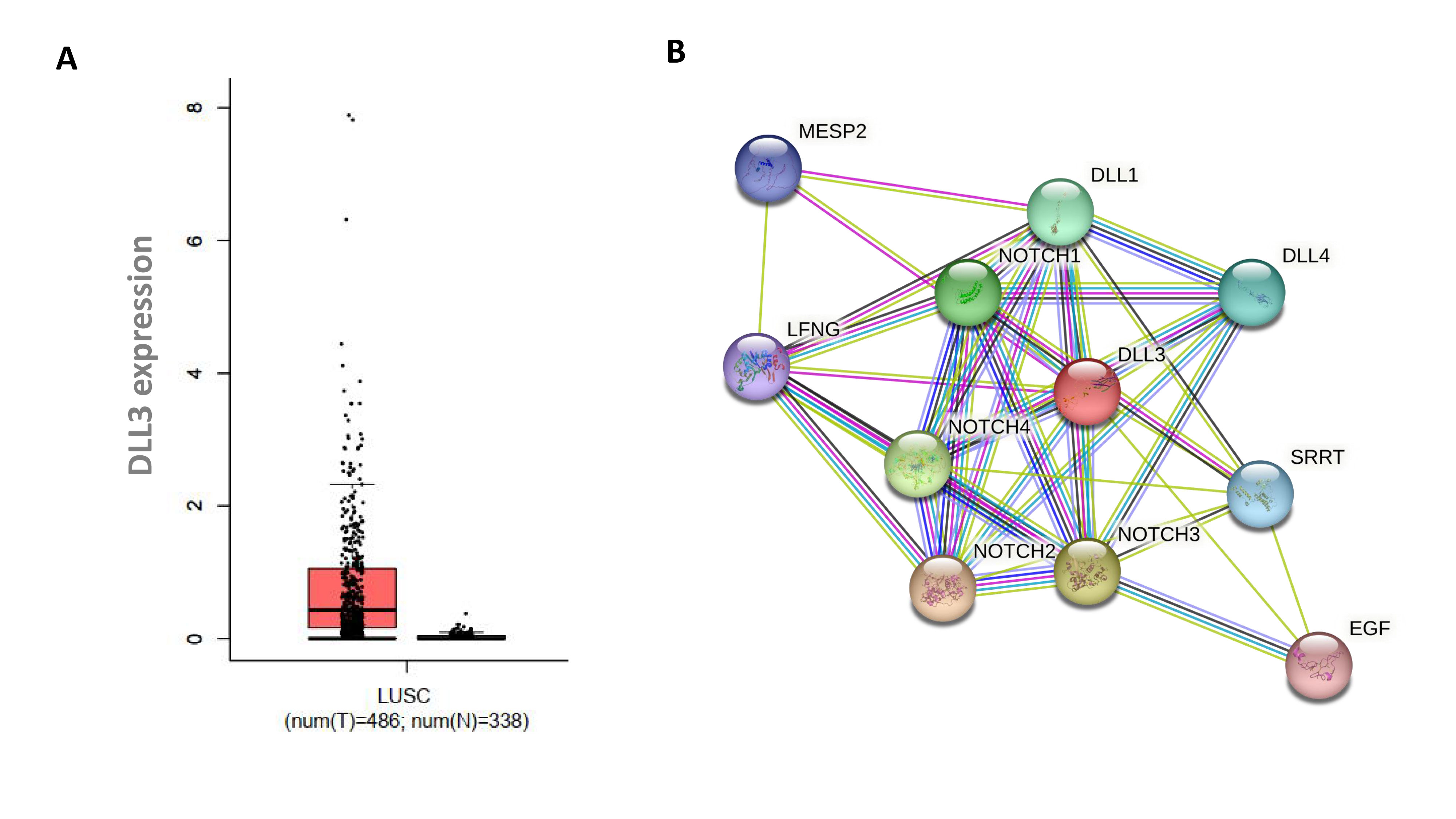 Figure 1. Notch signaling pathway. (A) Elevated DLL3 expression in Lung Squamous Cell Carcinoma (LUSC), Red bar represents DLL3 expression in LUSC tumor tissues and the black bar represents DLL3 expression in normal tissues; (B) DLL3 signaling, which is a central player of the Notch signaling pathway.
Figure 1. Notch signaling pathway. (A) Elevated DLL3 expression in Lung Squamous Cell Carcinoma (LUSC), Red bar represents DLL3 expression in LUSC tumor tissues and the black bar represents DLL3 expression in normal tissues; (B) DLL3 signaling, which is a central player of the Notch signaling pathway.
Tarlatamab exerts potentially severe neurotoxicity, encompassing immune effector cell-associated neurotoxicity syndrome (ICANS). Pooled safety analyses revealed neurologic adverse events in 47% of treated patients, with 10% representing Grade 3 severity. The predominant manifestations included cephalgia (14%), peripheral neuropathy (7%), vertigo (7%), sleep initiation/maintenance disorder (6%), myasthenia (3.7%), acute confusional state (2.1%), transient loss of consciousness (1.6%), and generalized neurotoxicity (1.1%) [58].
Tarlatamab induces cytopenic events, neutropenia, thrombocytopenia, and anemia. Clinical safety data revealed, Neutrophil count reduction occurred in 12% of treated patients (6% Grade 3/4), with median onset of severe neutropenia at 29.5 days (range: 2-213). Thrombocytopenia manifested in 33% of cases (3.2% Grade 3/4), exhibiting a median latency of 50 days for severe presentations (range: 3-420). Hemoglobin decline was observed in 58% of recipients (5% Grade 3/4). Febrile neutropenia incidence was 0.5% [58].
Tarlatamab exerts potential hepatotoxic effects, as evidenced by pooled safety analyses, Alanine aminotransferase (ALT) elevations manifested in 42% of treated patients (2.1% Grade 3/4). Aspartate aminotransferase (AST) increases occurred in 44% of cases (3.2% Grade 3/4). Hyperbilirubinemia developed in 15% of recipients (1.6% Grade 3/4), severe Type I hypersensitivity reactions, characterized by immune-mediated manifestations including rash, and bronchospasm. Tarlatamab also may exert harmful effects to the fetus when administered to a pregnant woman [58].
Prior investigations by Hughes et al. demonstrated that tarlatamab exhibited potent cytotoxic activity against SCLC cell lines, even those with minimal DLL3 expression (<1,000 surface molecules/cell). Mechanistic studies revealed that systemic administration of tarlatamab induced robust T-cell activation and mediated targeted tumor cell lysis through immune synapse formation [20]. This activity translated to significant antitumor efficacy across multiple in vivo models, including complete regression in patient-derived xenografts (PDXs) of SCLC, orthotopic primary lung tumors, and metastatic hepatic lesions. Toxicology assessments showed favorable safety profiles at doses up to 4.5 mg/kg, which achieved systemic exposures surpassing the mean in vitro EC50 for T-cell engagement. Notably, no drug-related adverse effects were observed in these preclinical safety studies. Collectively, these findings support the clinical study of tarlatamab as a promising immunotherapeutic strategy for DLL3-expressing SCLC, with demonstrated efficacy at low antigen density and an acceptable preclinical safety window [20, 59].
In another clinical study was conducted by University of Virginia. The efficacy analysis cohort comprised exclusively of SCLC patients receiving tarlatamab, with exclusion of the atypical carcinoid case included in safety evaluations. Median therapy duration was 8 weeks (range: 1-35) with three median treatment. Treatment persistence: 22.7% (n=5) remained on therapy at data cutoff, including one patient continuing post-progression following stereotactic radiosurgery (SRS) [60]. Therapeutic outcomes demonstrated, ORR was 42.9% and rapid response kinetics was 88.8% of responses (n=8) manifested within 6 weeks of initiation Disease progression or mortality was 66.6% (n=14) during follow-up. Exploratory biomarker analysis (n=18) suggested: LDH reduction post-cycle 1 correlated with enhanced disease control probability (stable disease/partial response; OR=9, p=0.12) Median progression-free survival (mPFS): 2.7 months [60].
|
Table 2. Pharmacokinetic parameters of tarlatamab[61] |
||
|
Parameter |
Value (Mean ± SD or Geometric Mean [%CV]) |
Notes |
|
Bioavailability |
Not applicable (IV administration) |
Administered intravenously. |
|
Volume of Distribution (Vd) |
~5.2–6.8 L |
Suggests limited distribution beyond plasma. |
|
Clearance (CL) |
~0.64 L/day |
Non-linear clearance at lower doses; linear at higher doses. |
|
Half-life (t½) |
~5.8 days |
Supports every 2- or 4-week dosing regimens. |
|
Cmax |
Dose-dependent |
Increases proportionally with dose (e.g., 1 mg/kg to 100 mg/kg). |
|
Tmax |
End of infusion |
Immediate peak post-IV administration. |
|
Area Under Curve (AUC) |
Dose-proportional |
Non-linear PK at low doses; linear at therapeutic doses (>10 mg/kg). |
|
Immunogenicity |
~10–20% ADA incidence |
Anti-drug antibodies may affect exposure in a subset of patients. |
Tarlatamab construct integrating an immunoglobulin G crystallizable fragment (Fc) domain with an anti-DLL3 × anti-CD3 BiTE (bispecific T-cell engager) scaffold. The inclusion of an engineered, effector-function-silent Fc domain confers prolonged serum persistence, enabling reduced dosing frequency [62]. Tarlatamab exhibits high-affinity binding to human DLL3 [equilibrium dissociation constant (K) = 0.64 nM] and CD3 (K = 14.9 nM), demonstrating potent in vitro cytotoxicity against DLL3-positive small cell lung carcinoma (SCLC) cell lines—even those with low antigen density (<1000 molecules/cell)—as well as DLL3-expressing prostate adenocarcinoma models [63].
Mechanistically, tarlatamab activates CD3 T lymphocytes, stimulating proinflammatory cytokine secretion and eliciting T-cell-mediated lysis of SCLC cells, DLL3 prostate cancer cells in vitro, and small-cell/neuroendocrine (SCNC) prostate cancer patient-derived xenograft (PDX) cells ex vivo. Notably, in vitro assays revealed rapid, target-dependent cytotoxicity against DLL3-expressing tumors, with minimal off-target effects on DLL3-negative bystander cells. Tarlatamab demonstrated comparable cytotoxic efficacy against both treatment-naïve and chemoresistant small cell lung carcinoma (SCLC) cell lines, indicating its therapeutic potential for relapsed/refractory disease. Synergistic enhancement of tumor cell killing was observed when tarlatamab was co-administered with platinum-based chemotherapeutics, etoposide, or combination regimens. Mechanistically, tarlatamab upregulated programmed death-ligand 1 (PD-L1) expression on SCLC cells, which potentiated the cytotoxic effects when combined with PD-1/PD-L1 axis inhibitors in vitro [64]. These findings support the clinical investigation of tarlatamab as a combination therapeutic with existing standard-of-care (SOC) regimens for SCLC management [62].
Tarlatamab, binds with DLL3 on the tumor cell surface, and CD3 on T-cells simultaneously leading to form immune synapse resulting cytotoxic T-cell-mediated tumor cell eradication [17]. In vitro studies demonstrate that tarlatamab mediates robust T-cell activation upon engagement with DLL3-positive SCLC cell lines, culminating in targeted tumor cell lysis through granzyme/perforin-mediated cytotoxicity (Figure 2). This antitumor activity translates to significant in vivo efficacy, with tarlatamab inducing substantial tumor regression in disseminated orthotopic SCLC models that recapitulate human disease progression. As the first DLL3-directed immunotherapeutic agent to enter clinical evaluation, tarlatamab represents a novel therapeutic strategy for SCLC, a malignancy historically refractory to conventional treatments [18].
The notch signaling cascade serves as a master regulator of developmental ontogeny, including pulmonary neuroendocrine cell differentiation [70]. DLL3, a transmembrane notch pathway antagonist, exhibits strict intracellular localization in healthy adult tissues, primarily restricted to Golgi compartments [59, 71]. This inhibitory ligand represents a direct transcriptional target of achaete-scute homolog 1 (ASCL1), a basic helix-loop-helix transcription factor that drives neuroendocrine proliferation and is fundamentally implicated in small cell lung carcinoma tumorigenesis [72-74]. In ASCL1-positive SCLC, DLL3 undergoes profound overexpression coupled with aberrant cell surface translocation, creating a tumor-specific epitope that presents an ideal therapeutic target for precision oncology approaches [48, 75, 76].
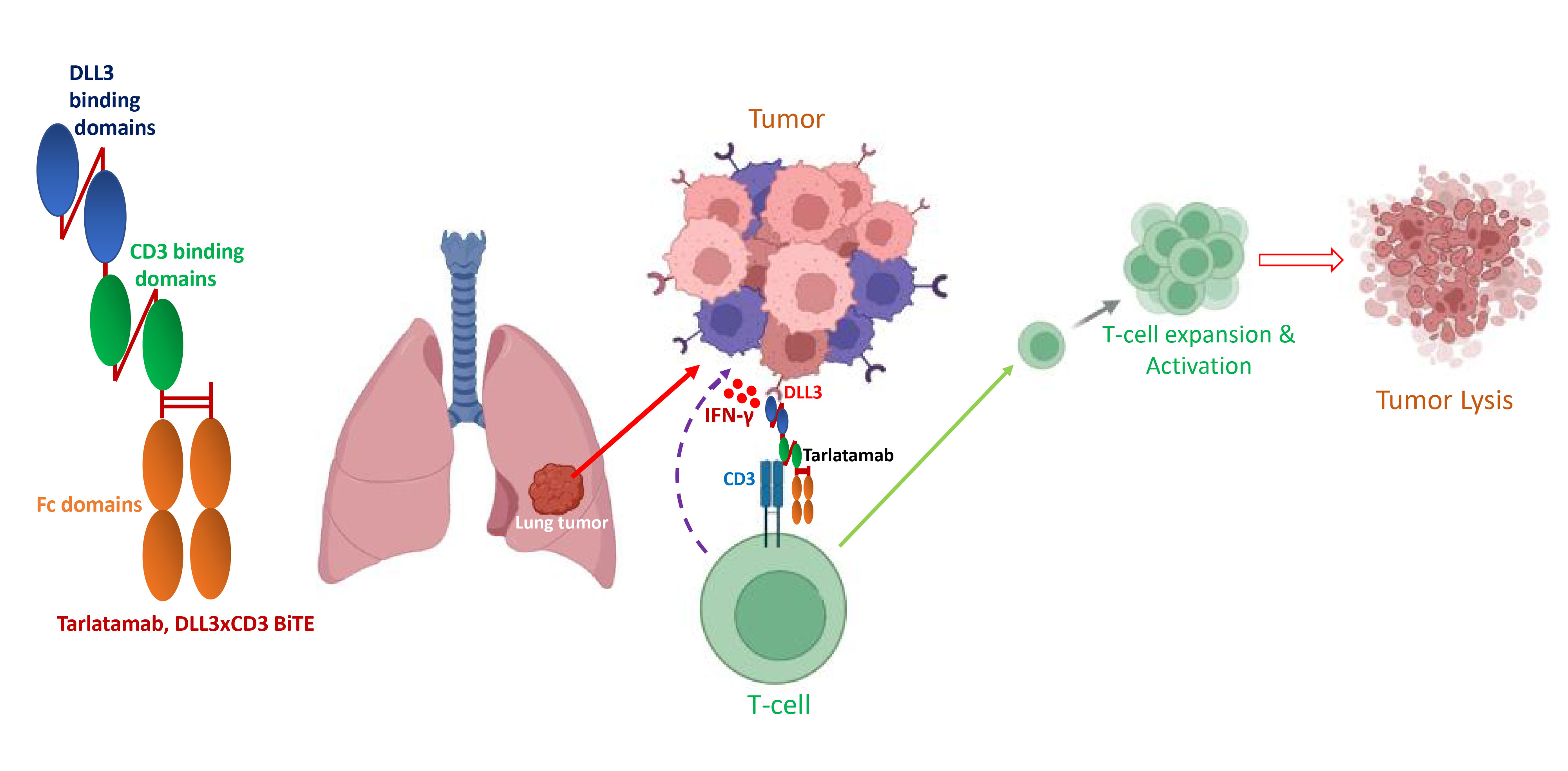 Figure 2. Schematic diagram of Tarlatamab, and its T-cell-mediated mechanism of tumor lysis. Tarlatamab comprised DLL3 and CD3 binding domains. It binds with T-cells through CD3 binding domains and targets DLL3 through DLL3 binding domains simultaneously. As a result, activated T-cells are redirected to the DLL3-overexpressed tumor cells leading to potential actions of tumor lysis.
Figure 2. Schematic diagram of Tarlatamab, and its T-cell-mediated mechanism of tumor lysis. Tarlatamab comprised DLL3 and CD3 binding domains. It binds with T-cells through CD3 binding domains and targets DLL3 through DLL3 binding domains simultaneously. As a result, activated T-cells are redirected to the DLL3-overexpressed tumor cells leading to potential actions of tumor lysis.
Given prior evidence implicating notch signaling in SCLC pathogenesis and DLL3 regulation [79], transcriptional changes in this pathway were evaluated in pre- and post-tarlatamab tumors. When compared to the four reference cell lines, post-treatment tumors demonstrated upregulation of notch family genes and downregulation of DELTA-like ligands. This shift toward a notch-receptive phenotype, consistent with lateral inhibition dynamics, supports the hypothesis that tarlatamab resistance may arise through notch-mediated suppression of DLL3 [80]. A sharp differential expression among the four SCLC subtypes was observed through the adapted nCounter assay for molecular subtyping; however, comprehensive validation of these transcriptional changes following tarlatamab treatment may require RNA sequencing of the entire transcriptome across large clinical cohorts. The development of a clinically feasible and robust SCLC subtyping methodology would facilitate broader investigations into the influence of molecular subtypes on tarlatamab therapeutic efficacy. Findings from such studies are anticipated to optimize tarlatamab responsiveness and inform the development of novel strategies to circumvent resistance mechanisms [81].
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
Ahmed Attia Ahmed Abdelmoaty and contributed to draft, critical revision of the article, table making, figure drawing and final submission. Ahmed Gamal Badran draw the figures and revised the manuscript.
Competing interests
The authors declare no competing interests.
- Wang S, Tang J, Sun T, Zheng X, Li J, Sun H, Zhou X, Zhou C, Zhang H, Cheng Z et al: Survival changes in patients with small cell lung cancer and disparities between different sexes, socioeconomic statuses and ages. Sci Rep 2017, 7(1): 1339.
- Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ: The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med 2020, 383(7): 640-649.
- Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC et al: Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open 2022, 7(2): 100408.
- Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J, Califano R, Nishio M et al: Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol 2021, 39(6): 619-630.
- von Pawel J, Schiller JH, Shepherd FA, Fields SZ, Kleisbauer JP, Chrysson NG, Stewart DJ, Clark PI, Palmer MC, Depierre A et al: Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 1999, 17(2): 658-667.
- Petrelli F, Ghidini A, Luciani A: Topotecan or other agents as second-line therapy for relapsed small-cell lung cancer: A meta-analysis of randomized studies. Mol Clin Oncol 2021, 15(4): 218.
- Baena J, Modrego A, Zeaiter A, Kahatt C, Alfaro V, Jimenez-Aguilar E, Mazarico JM, Paz-Ares L: Lurbinectedin in the treatment of relapsed small cell lung cancer. Future Oncol 2021, 17(18): 2279-2289.
- Trigo J, Subbiah V, Besse B, Moreno V, López R, Sala MA, Peters S, Ponce S, Fernández C, Alfaro V et al: Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol 2020, 21(5): 645-654.
- Guo Q, Liu L, Chen Z, Fan Y, Zhou Y, Yuan Z, Zhang W: Current treatments for non-small cell lung cancer. Front Oncol 2022, 12: 945102.
- Mahadevan NR, Knelson EH, Wolff JO, Vajdi A, Saigí M, Campisi M, Hong D, Thai TC, Piel B, Han S et al: Intrinsic Immunogenicity of Small Cell Lung Carcinoma Revealed by Its Cellular Plasticity. Cancer Discov 2021, 11(8): 1952-1969.
- Cornel AM, Mimpen IL, Nierkens S: MHC Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers (Basel) 2020, 12(7): 1760.
- van de Donk N, Zweegman S: T-cell-engaging bispecific antibodies in cancer. Lancet 2023, 402(10396): 142-158.
- Haber L, Olson K, Kelly MP, Crawford A, DiLillo DJ, Tavaré R, Ullman E, Mao S, Canova L, Sineshchekova O et al: Generation of T-cell-redirecting bispecific antibodies with differentiated profiles of cytokine release and biodistribution by CD3 affinity tuning. Scientific Reports 2021, 11(1): 14397.
- Wu Z, Cheung NV: T cell engaging bispecific antibody (T-BsAb): From technology to therapeutics. Pharmacol Ther 2018, 182: 161-175.
- McCue AC, Demarest SJ, Froning KJ, Hickey MJ, Antonysamy S, Kuhlman B: Engineering a tumor-selective prodrug T-cell engager bispecific antibody for safer immunotherapy. MAbs 2024, 16(1): 2373325.
- Tada M, Aoyama M, Ishii-Watabe A: Target-independent Immune-cell Activation by Aggregates of T Cell-redirecting Bispecific Antibodies. Journal of Pharmaceutical Sciences 2023, 112(9): 2419-2426.
- Giffin MJ, Cooke K, Lobenhofer EK, Estrada J, Zhan J, Deegen P, Thomas M, Murawsky CM, Werner J, Liu S et al: AMG 757, a Half-Life Extended, DLL3-Targeted Bispecific T-Cell Engager, Shows High Potency and Sensitivity in Preclinical Models of Small-Cell Lung Cancer. Clin Cancer Res 2021, 27(5): 1526-1537.
- Giffin MJ, Lobenhofer EK, Cooke KS, Raum T, Stevens JL, Beltran PJ, Coxon A, Hughes PE: Abstract 3632: BiTE® antibody constructs for the treatment of SCLC. Cancer Research 2017, 77: 3632-3632.
- Owen DH, Giffin MJ, Bailis JM, Smit MD, Carbone DP, He K: DLL3: an emerging target in small cell lung cancer. J Hematol Oncol 2019, 12(1): 61.
- Tang D, Kang R: Tarlatamab: the promising immunotherapy on its way from the lab to the clinic. Transl Lung Cancer Res 2023, 12(6): 1355-1357.
- Khosla AA, Jatwani K, Singh R, Reddy A, Jaiyesimi I, Desai A: Bispecific Antibodies in Lung Cancer: A State-of-the-Art Review. Pharmaceuticals (Basel) 2023, 16(10): 1461.
- Oronsky B, Abrouk N, Caroen S, Lybeck M, Guo X, Wang X, Yu Z, Reid T: A 2022 Update on Extensive Stage Small-Cell Lung Cancer (SCLC). J Cancer 2022, 13(9): 2945-2953.
- Sabari JK, Lok BH, Laird JH, Poirier JT, Rudin CM: Unravelling the biology of SCLC: implications for therapy. Nat Rev Clin Oncol 2017, 14(9): 549-561.
- Ahn MJ, Cho BC, Felip E, Korantzis I, Ohashi K, Majem M, Juan-Vidal O, Handzhiev S, Izumi H, Lee JS et al: Tarlatamab for Patients with Previously Treated Small-Cell Lung Cancer. N Engl J Med 2023, 389(22): 2063-2075.
- Frampton JE: Epcoritamab: First Approval. Drugs 2023, 83(14): 1331-1340.
- Epcoritamab (Epkinly) for diffuse large B-cell lymphoma (DLBCL). Med Lett Drugs Ther 2023, 65(1678): e103-e104.
- Wu X, Yang X, Dai Y, Zhao Z, Zhu J, Guo H, Yang R: Single-cell sequencing to multi-omics: technologies and applications. Biomarker Research 2024, 12(1): 110.
- Paz-Ares L, Champiat S, Lai WV, Izumi H, Govindan R, Boyer M, Hummel HD, Borghaei H, Johnson ML, Steeghs N et al: Tarlatamab, a First-in-Class DLL3-Targeted Bispecific T-Cell Engager, in Recurrent Small-Cell Lung Cancer: An Open-Label, Phase I Study. J Clin Oncol 2023, 41(16): 2893-2903.
- Rais T, Khan A, Riaz R: Elrexfio™ (elranatamab-bcmm): The game-changer in treatment of multiple myeloma. Rare Tumors 2023, 15: 20363613231207483.
- Lu Y, Li M, Gao Z, Ma H, Chong Y, Hong J, Wu J, Wu D, Xi D, Deng W: Innovative Insights into Single-Cell Technologies and Multi-Omics Integration in Livestock and Poultry. International Journal of Molecular Sciences 2024, 25(23): 12940.
- Dhillon S: Elranatamab: First Approval. Drugs 2023, 83(17): 1621-1627.
- Passaro A, Wang J, Wang Y, Lee SH, Melosky B, Shih JY, Wang J, Azuma K, Juan-Vidal O, Cobo M et al: Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol 2024, 35(1): 77-90.
- Cho BC, Lu S, Felip E, Spira AI, Girard N, Lee JS, Lee SH, Ostapenko Y, Danchaivijitr P, Liu B et al: Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N Engl J Med 2024, 391(16): 1486-1498.
- Okajima D, Yasuda S, Maejima T, Karibe T, Sakurai K, Aida T, Toki T, Yamaguchi J, Kitamura M, Kamei R et al: Datopotamab Deruxtecan, a Novel TROP2-directed Antibody-drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol Cancer Ther 2021, 20(12): 2329-2340.
- Bardia A, Jhaveri K, Kalinsky K, Pernas S, Tsurutani J, Xu B, Hamilton E, Im SA, Nowecki Z, Sohn J et al: TROPION-Breast01: Datopotamab deruxtecan vs chemotherapy in pre-treated inoperable or metastatic HR+/HER2- breast cancer. Future Oncol 2024, 20(8): 423-436.
- Shimizu T, Sands J, Yoh K, Spira A, Garon EB, Kitazono S, Johnson ML, Meric-Bernstam F, Tolcher AW, Yamamoto N et al: First-in-Human, Phase I Dose-Escalation and Dose-Expansion Study of Trophoblast Cell-Surface Antigen 2-Directed Antibody-Drug Conjugate Datopotamab Deruxtecan in Non-Small-Cell Lung Cancer: TROPION-PanTumor01. J Clin Oncol 2023, 41(29): 4678-4687.
- Dent RA, Cescon DW, Bachelot T, Jung KH, Shao ZM, Saji S, Traina TA, Vukovic P, Mapiye D, Maxwell MJ et al: TROPION-Breast02: Datopotamab deruxtecan for locally recurrent inoperable or metastatic triple-negative breast cancer. Future Oncol 2023, 19(35): 2349-2359.
- Shitara K, Lordick F, Bang YJ, Enzinger P, Ilson D, Shah MA, Van Cutsem E, Xu RH, Aprile G, Xu J et al: Zolbetuximab plus mFOLFOX6 in patients with CLDN18.2-positive, HER2-negative, untreated, locally advanced unresectable or metastatic gastric or gastro-oesophageal junction adenocarcinoma (SPOTLIGHT): a multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 401(10389): 1655-1668.
- Shah MA, Shitara K, Ajani JA, Bang YJ, Enzinger P, Ilson D, Lordick F, Van Cutsem E, Gallego Plazas J, Huang J et al: Zolbetuximab plus CAPOX in CLDN18.2-positive gastric or gastroesophageal junction adenocarcinoma: the randomized, phase 3 GLOW trial. Nat Med 2023, 29(8): 2133-2141.
- Keam SJ: Zolbetuximab: First Approval. Drugs 2024, 84(8): 977-983.
- Kubota Y, Shitara K: Zolbetuximab for Claudin18.2-positive gastric or gastroesophageal junction cancer. Ther Adv Med Oncol 2024, 16: 17588359231217967.
- Necchi A, Pouessel D, Leibowitz R, Gupta S, Fléchon A, García-Donas J, Bilen MA, Debruyne PR, Milowsky MI, Friedlander T et al: Pemigatinib for metastatic or surgically unresectable urothelial carcinoma with FGF/FGFR genomic alterations: final results from FIGHT-201. Ann Oncol 2024, 35(2): 200-210.
- Sahin U, Türeci Ö, Manikhas G, Lordick F, Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B et al: FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 2021, 32(5): 609-619.
- Klempner SJ, Lee KW, Shitara K, Metges JP, Lonardi S, Ilson DH, Fazio N, Kim TY, Bai LY, Moran D et al: ILUSTRO: Phase II Multicohort Trial of Zolbetuximab in Patients with Advanced or Metastatic Claudin 18.2-Positive Gastric or Gastroesophageal Junction Adenocarcinoma. Clin Cancer Res 2023, 29(19): 3882-3891.
- Zolbetuximab. In: Drugs and Lactation Database (LactMed®). Epub ahead of print., edn. Bethesda (MD): National Institute of Child Health and Human Development; 2006.
- George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, Leenders F, Lu X, Fernández-Cuesta L, Bosco G et al: Comprehensive genomic profiles of small cell lung cancer. Nature 2015, 524(7563): 47-53.
- Lim JS, Ibaseta A, Fischer MM, Cancilla B, O'Young G, Cristea S, Luca VC, Yang D, Jahchan NS, Hamard C et al: Intratumoural heterogeneity generated by Notch signalling promotes small-cell lung cancer. Nature 2017, 545(7654): 360-364.
- Saunders LR, Bankovich AJ, Anderson WC, Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang A et al: A DLL3-targeted antibody-drug conjugate eradicates high-grade pulmonary neuroendocrine tumor-initiating cells in vivo. Sci Transl Med 2015, 7(302): 302ra136.
- Tanaka K, Isse K, Fujihira T, Takenoyama M, Saunders L, Bheddah S, Nakanishi Y, Okamoto I: Prevalence of Delta-like protein 3 expression in patients with small cell lung cancer. Lung Cancer 2018, 115: 116-120.
- Huang RSP, Holmes BF, Powell C, Marati RV, Tyree D, Admire B, Streator A, Newell AEH, Perez J, Dalvi D et al: Delta-like Protein 3 Prevalence in Small Cell Lung Cancer and DLL3 (SP347) Assay Characteristics. Arch Pathol Lab Med 2019, 143(11): 1373-1377.
- Rojo F, Corassa M, Mavroudis D, Öz AB, Biesma B, Brcic L, Pauwels P, Sailer V, Gosney J, Miljkovic D et al: International real-world study of DLL3 expression in patients with small cell lung cancer. Lung Cancer 2020, 147: 237-243.
- Ding J, Yeong C: Advances in DLL3-targeted therapies for small cell lung cancer: challenges, opportunities, and future directions. Frontiers in Oncology 2024, 14: 1504139.
- Furuta M, Kikuchi H, Shoji T, Takashima Y, Kikuchi E, Kikuchi J, Kinoshita I, Dosaka-Akita H, Sakakibara-Konishi J: DLL3 regulates the migration and invasion of small cell lung cancer by modulating Snail. Cancer Sci 2019, 110(5): 1599-1608.
- Rudin CM, Pietanza MC, Bauer TM, Ready N, Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA, 3rd, Robert F et al: Rovalpituzumab tesirine, a DLL3-targeted antibody-drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol 2017, 18(1): 42-51.
- Trials C: Tarlatamab Clinical Trial Listings. wwwclinicaltrialsgov March 15, 2024. Epub ahead of print.
- Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K: Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Molecular Cancer 2022, 21(1): 28.
- Shen S, Hong Y, Huang J, Qu X, Sooranna SR, Lu S, Li T, Niu B: Targeting PD-1/PD-L1 in tumor immunotherapy: Mechanisms and interactions with host growth regulatory pathways. Cytokine & Growth Factor Reviews 2024, 79: 16-28.
- https://www.amgen.com/newsroom/press-releases/2024/05/fda-approves-imdelltra-tarlatamabdlle-the-first-and-only-tcell-engager-therapy-for-the-treatment-of-extensivestage-small-cell-lung-cancer
- Zhang H, Yang Y, Li X, Yuan X, Chu Q: Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. Biomedicine & Pharmacotherapy 2023, 159: 114248.
- Bolte FJ, Dougherty SC, Danos AO, Lynch AC, Shvorak Y, Statler S, Gentzler RD, Hall RD: Real-World Outcomes of Tarlatamab in Small Cell Lung Cancer, Including Patients with Untreated Brain Metastases. Clinical Lung Cancer 2025, https://doi.org/https://doi.org/10.1016/j.cllc.2025.03.006. Epub ahead of print.
- Minocha M, Thompson CG, Murphy A, Zhou Y, Brandl C, Parkes A, Chen X, Yu B, Martinez P, Houk BE: Pharmacokinetics of Tarlatamab, a Delta-Like Ligand-3 (DLL3) Targeted Half-Life Extended Bispecific T-Cell Engager (BiTE(®)) Immunotherapy in Adult Patients with Previously Treated Small-Cell Lung Cancer: Results from DeLLphi-300, a Phase I Multiple-Dose-Escalation Study. Clin Pharmacokinet 2024, 63(12): 1757-1768.
- Dhillon S: Tarlatamab: First Approval. Drugs 2024, 84(8): 995-1003.
- Chou J, Egusa EA, Wang S, Badura ML, Lee F, Bidkar AP, Zhu J, Shenoy T, Trepka K, Robinson TM et al: Immunotherapeutic Targeting and PET Imaging of DLL3 in Small-Cell Neuroendocrine Prostate Cancer. Cancer Res 2023, 83(2): 301-315.
- You R, Estrada J, Zhan J, Deegen P, Cooke K, Friedrich M, Bailis JM: 1189 Elucidating the effects of chemotherapy and immune checkpoint blockade on the activity of tarlatamab, a DLL3-targeting bispecific T cell engager molecule, in small cell lung cancer preclinical models. Journal for ImmunoTherapy of Cancer 2023, 11(Suppl 1): A1309-A1309.
- Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G: Harnessing T cells to fight cancer with BiTE® antibody constructs--past developments and future directions. Immunol Rev 2016, 270(1): 193-208.
- Yuraszeck T, Kasichayanula S, Benjamin JE: Translation and Clinical Development of Bispecific T-cell Engaging Antibodies for Cancer Treatment. Clin Pharmacol Ther 2017, 101(5): 634-645.
- Li G, Reid KM, Spitler K, Beatty N, Boucher J, Davila ML: CD3 engagement as a new strategy for allogeneic "off-the-shelf" T cell therapy. Mol Ther Oncolytics 2022, 24: 887-896.
- Banaszek A, Bumm TGP, Nowotny B, Geis M, Jacob K, Wölfl M, Trebing J, Kucka K, Kouhestani D, Gogishvili T et al: On-target restoration of a split T cell-engaging antibody for precision immunotherapy. Nature Communications 2019, 10(1): 5387.
- Duell J, Lammers PE, Djuretic I, Chunyk AG, Alekar S, Jacobs I, Gill S: Bispecific Antibodies in the Treatment of Hematologic Malignancies. Clin Pharmacol Ther 2019, 106(4): 781-791.
- Morimoto M, Nishinakamura R, Saga Y, Kopan R: Different assemblies of Notch receptors coordinate the distribution of the major bronchial Clara, ciliated and neuroendocrine cells. Development 2012, 139(23): 4365-4373.
- Peddio A, Pietroluongo E, Lamia MR, Luciano A, Caltavituro A, Buonaiuto R, Pecoraro G, De Placido P, Palmieri G, Bianco R et al: DLL3 as a potential diagnostic and therapeutic target in neuroendocrine neoplasms: A narrative review. Critical Reviews in Oncology/Hematology 2024, 204: 104524.
- Augustyn A, Borromeo M, Wang T, Fujimoto J, Shao C, Dospoy PD, Lee V, Tan C, Sullivan JP, Larsen JE et al: ASCL1 is a lineage oncogene providing therapeutic targets for high-grade neuroendocrine lung cancers. Proc Natl Acad Sci U S A 2014, 111(41): 14788-14793.
- Olsen RR, Ireland AS, Kastner DW, Groves SM, Spainhower KB, Pozo K, Kelenis DP, Whitney CP, Guthrie MR, Wait SJ et al: ASCL1 represses a SOX9(+) neural crest stem-like state in small cell lung cancer. Genes Dev 2021, 35(11-12): 847-869.
- Tenjin Y, Kudoh S, Kubota S, Yamada T, Matsuo A, Sato Y, Ichimura T, Kohrogi H, Sashida G, Sakagami T et al: Ascl1-induced Wnt11 regulates neuroendocrine differentiation, cell proliferation, and E-cadherin expression in small-cell lung cancer and Wnt11 regulates small-cell lung cancer biology. Laboratory Investigation 2019, 99(11): 1622-1635.
- Hu C, Dong J, Liu L, Liu J, Sun X, Teng F, Wang X, Ying J, Li J, Xing P et al: ASCL1 and DLL3 expressions and their clinicopathological implications in surgically resected pure small cell lung cancer: A study of 247 cases from the National Cancer Center of China. Thorac Cancer 2022, 13(3): 338-345.
- Hu C, Dong J, Liu L, Liu J, Sun X, Teng F, Wang X, Ying J, Li J, Xing P et al: ASCL1 and DLL3 expressions and their clinicopathological implications in surgically resected pure small cell lung cancer: A study of 247 cases from the National Cancer Center of China. Thoracic Cancer 2022, 13(3): 338-345.
- Amgen: https://pdf.hres.ca/dpd_pm/00077062.PDF. 2024Epub ahead of print.
- Gay CM, Stewart CA, Park EM, Diao L, Groves SM, Heeke S, Nabet BY, Fujimoto J, Solis LM, Lu W et al: Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell 2021, 39(3): 346-360.e347.
- Zhang H, Yang Y, Li X, Yuan X, Chu Q: Targeting the Notch signaling pathway and the Notch ligand, DLL3, in small cell lung cancer. Biomed Pharmacother 2023, 159: 114248.
- Kim JW, Ko JH, Sage J: DLL3 regulates Notch signaling in small cell lung cancer. iScience 2022, 25(12): 105603.
- Ahn HM, Park SY, Choi Y, Kim J, Lee Y: Molecular subtype changes after acquiring resistance to tarlatamab in small cell lung cancer. Drug Resist Updat 2025, 79: 101198.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript