Research Article | Open Access
Rapamycin reduces testicular ischemia-reperfusion injury by enhancing autophagy
Zhi Hu1, *, Qiong Cheng2, *, Lv Xu1, Yiyan Chen1, Jinzuo Ning3, Fan Cheng3, Wei Zhang1
1Department of Urology, Tongren Hospital of Wuhan University, Wuhan Third Hospital, Wuhan, Hubei 430060, China.
2Department of Medical Ultrasonics, Hubei Maternal and Child Health Hospital, Wuhan, Hubei 430060, China.
3Department of Urology, Renmin Hospital of Wuhan University, Wuhan, Hubei 430060, China.
*: These authors equally contribute to the works.
Correspondence: Wei Zhang (Department of Urology, Tongren Hospital of Wuhan University, Wuhan Third Hospital, Wuhan, Hubei 430060, China; E-mail: 1006847164@qq.com).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2024, 4: 26-33. https://doi.org/10.32948/ajpt.2024.05.13
Received: 04 May 2024 | Accepted: 14 May 2024 | Published online: 17 May 2024
Methods Forty rats were divided into sham group, I/R group, I/R+Rap (rapamycin, autophagy activator) group and I/R+ 3-MA (3-methyl adenine, autophagy inhibitor) group. Before inducing ischemia, rapamycin and 3-MA were intraperitoneally injected into I/R+Rap and I/R+ 3-ma groups, respectively. Subsequently, we then assessed testicular tissue damage. Immunohistochemistry was used to detect Beclin-1 and Caspase-3, while Western blot and qRT-PCR detected LC-II, Beclin-1 and p62. TUNEL and transmission electron microscopy were used to observe apoptosis and autophagosome.
Results I/R activated autophagy in rat testicles. Rapamycin significantly improved testicular function after I/R by enhancing autophagy, reducing spermatogenic cell apoptosis, and decreasing testicular tissue damage scores.
Conclusions Enhancing autophagy has a protective effect in ischemic-reperfusion injury by reducing apoptosis of rat testicular sperm cells.
Key words autophagy, ischemia-reperfusion, testicular torsion, rapamycin, 3-methyladenine
Autophagy, present in all eukaryotes, is an intracellular degradation system regulated by autophagy-associated proteins (ATG), through which cellular material is delivered to and degraded in lysosomes. However, autophagy not only removes material components, but also repairs cells and maintains homeostatic production in the body. Autophagy is a complex cytoplasmic component degradation process that is upregulated during starvation to counteract nutrient deprivation [5]. It is programmed cell death of type II [6], which can be activated by various physiological and pathological factors to promote cell survival or lead to cell death [7, 8]. Autophagy serves as a defensive mechanism against environmental stress and is essential for various physiological and pathological processes. It is an induced and regulated process that determines cell survival or death in various ways [9]. Experiments have shown that I/R induced damage effectively triggers autophagy factors, and high levels of authophagy under high atherosclerotic shear stress might inhibit endothelial cell death and inflammation, thereby preventing atherosclerosis [10]. Microtubule- associated proteins 1A and 1B (LC3) and Beclin-1 are involved in autophagy. Treatment of LC3I to LC3II is related to the degree of authophagic granule formation. LC3II serves as an autophagic marker [11, 12]. Autophagy effectively degrades the autophagy protein p62, and the level of p62 negatively correlates with autophagy activity13. Apoptosis increases with autophagy inactivation, suggesting that increased autophagy can inhibit apoptosis [14].
This study regulates autophagy using rapamycin and 3-MA to explore its role in rat models with testicular I/R injury. Rapamycin, a macrolide antibiotic, that induces autophagy through inhibiting mTOR [15, 16]. 3-MA is PI3K inhibitor that inhibits the formation of primary autophagic vesicles and autophagosomes [17].
We studied activated autophagy in testicular I/R injury. 3-MA may aggravate testicular tissue damage and increase apoptosis, while rapamycin increases autophagy and reduces apoptosis. These mechanisms and the regulation of LC3II, Beclin-1, p62 and Caspase-3 were investigated.
Male SD rats (200-250g/animal, n=10 for each group) were kept under a 12-hour light cycle at 20°C-22°C with a dark cycle. Animals were Intraperitoneal injected with of pentobarbital anaesthetized (45 mg/kg). Then, the rats were placed on a constant temperature operating board (37°C). During surgery, the scrotal skin was opened to release the left testicle. The left testicle was rotated clockwise 720° 1 hour, and four hours after reperfusion, all the rats were euthanized, and left testicular tissue was collected. Forty rats were divided into 4 groups (n=10): sham operation group (scrotal opening without testicular torsion), I/R group (the left testis rotated clockwise 720 ° for 1 hour, followed by four hours reperfusion), I/R+Rap group (rats given a tail vein injection of rapamycin (1 mg/kg) 15 minutes before torsion), I/R+ 3-MA group (3-MA(30 mg/kg) injected into tail vein 15 minutes before torsion, as previously described. After the experiment ended, the left testis was removed under anaesthesia, fixed in 10% phosphate-buffered formalin, and stored at -80 °C for further study.
Hematoxylin and eosin (HE) staining
After fixation with 4% paraformaldehyde, the paraffin blocks of renal tissue were cut into 5 mm thickness, and the sections were deparaffinized in xylene and dehydrated in alcohol. Then, they were stained in HE method.
Immunohistochemistry
Immunohistochemical staining kits were used to detect LC3-II and Caspase-3. After dehydration, the tissues were fixed with paraffin wax and sliced (4μm). The sections were deparaffinized, hydrated, followed by thermal antigen retrieval and blocking of endogenous peroxidases with 3% hydrogen peroxide. 4% goat serum was used to minimize non-specific staining. Samples were incubated with Beclin-1 and Caspase 3 antibodies (1: 200) at 4 ℃ overnight, followed by a 37 ℃ second incubation for 30 minutes. Each section was then treated with DAB chromogenic fluid. Hematoxylin was then used for counterstaining. Each sample was randomly selected from 5 different fields (400×) and the integrated optical density ratio (IOD) values were analyzed using image analysis software IP win32 (Acromag, Inc., Wixom, MI, USA) .
TUNEL staining
Apoptosis of spermatogonial cells was detected by TUNEL-mediated dUTP Nick terminal marker (TUNEL). The apoptosis detection kit was purchased from Roche, and the apoptotic nuclei were stained brown (DAB color). The negative control group used end transferase instead of PBS. The apoptosis index (AI) was calculated: 500 cells were counted to obtain the rate of positive cells.AI = 100% of positive cells/total cells.
Transmission electron microscopy
Approximately 1 mm3 of testicular tissue was taken from each animal, fixed at 4 ° C, washed, fixated, dehydrated, soaked, embedded, and sliced (60-90 nm each). Autophagosome ultrastructures were observed under transmission electron microscopy (Hitachi, Ltd., Tokyo, Japan).
Western blot
Total protein was extracted from testicular tissue according to manufacturer’s protocol using a Thermo Fisher Scientific reagent for mammalian proteins and quantified using a protein analysis kit (Hercules, Bio Rad, ca, USA) The proteins were electrophoretically separated, transferred to a PVDF membrane, and blocked with 5% nonfat emulsion at room temperature in Tris-buffered saline and Tween 20 buffer for 2 hours. The membrane was then incubated with primary antibodies (Cleaved Caspase 3 (CST, 1: 1000, #9664); LC3-II (CST, 1: 1000, #3868); Beclin-1 (CST, 1: 2000, #3495); P62 (Abcam, 1: 3000, ab109012); GAPDH (Abcam, 1: 1000, ab37168)) at 4°C overnight. After washing with TBST buffer, a secondary antibody (1: 2000, Wuhan DR Biological Engineering Co., Ltd.) was added and incubated at 37°C for 2 hours. The membrane was then washed, and the secondary antibody was detected for imaging.
RT-PCR
Total RNA was extracted and reversed into cDNA. The PCR reaction conditions were as follows: 10 min (94℃), 40 cycles of 15 s ( 94℃), 30 s (58℃), and 40 s (72℃). ABI 7500 and SYBR Green chemistry were used to detect LC3, Beclin-1, P62, and β-actin mRNA levels. The sequences are provided in Table 1. The PCR products were normalized to GAPDH levels, and the associated gene expression levels were calculated.
Statistical analysis
All data were analyzed using GraphPad Prism-7 and described as mean and standard deviations (mean ± SEM). One-way ANOVA was used to compare between groups. P<0.05 indicated statistical significance.
|
Table 1. PCR primer sequence. |
||
|
Gene |
Forward Primer (5`-3`) |
Reverse Primer (5`-3`) |
|
LC3II |
GAGTGGAAGATGTCCGGCTC |
GACACACTCACCATGCTGTGC |
|
Beclin-1 |
TCAATGCGACCTTCCATATCTG |
CTGTCAGGGACTCCAGATACGAG |
|
P62 |
GAAAGAGCGGGTACTGATCCC |
CCATAGCATGGGCCATAAGAG |
|
GAPDH |
CGCTAACATCAAATGGGGTG |
TTGCTGACAATCTTGAGGGAG |
Histological scores increased significantly after I/R injury (Figure 1 A(b)). To evaluate the activation level of autophagy, autophagy-related proteins LC3, Beclin-1 and p62 were detected by qRT-PCR and Western blot. After I/R injury, Beclin-1 positive cells increased (Figure 2). I/R injury also resulted in significant increase in LC3II and Beclin-1, and decreased expression of p62, but the decrease was not significant (Figure 3 and Figure 4). The structure of autophagic vacuoles was observed with a high-power (×5000) electron microscope, which is the gold standard for the detection of autophagy. Autophagic vacuoles were observed in the I/R group. (Figures 5 (A) and (B), white arrows). Taken together, these results confirm the occurrence of autophagy in this testicular I/R model. Apoptosis in testicular tissue is activated during I/R injury. TUNEL-positive cells were rarely observed in testicular tissue in the sham group (Figure 1A (e)). TUNEL-positive cells in testicular tissue sections increased in the I/R group compared to the sham group (Figure 1A (b)). Similarly, caspase-3 expression increased in the I/R group compared to the sham group (Figure 2).
MA aggravates testicular I/R damage, and rapamycin relieves testicular I/R damage
Immunohistochemical analysis showed that Beclin-1 and caspase-3 scores significantly increased compared with sham group. After rapamycin treatment, caspase-3 decreased significantly, while Beclin-1 was increased significantly. After 3-MA treatment, caspase-3 increased significantly, while Beclin-1 decreased significantly. (Figure 2) Compared with the sham group, LC3 and Beclin-1 mRNA expressions increased significantly, while p62 mRNA expression decreased significantly. After rapamycin treatment, LC3 and Beclin-1 mRNA expressions increased significantly, while the p62 mRNA expression was significantly decreased. After 3-MA treatment, LC3 and Beclin-1 mRNA expressions decreased significantly, while p62 mRNA expression increased significantly. (Figure 3) Western blot results showed that caspase-3, LC3, Beclin-1 and BNIP3 levels were increased significantly compared with sham group, while p62 level significantly decreased. After rapamycin treatment, caspase-3, LC3, Beclin-1 and BNIP3 levels was decreased significantly, while p62 level was increased significantly. After 3-MA treatment, LC3 and Beclin-1 levels increased significantly, while p62 level decreased significantly. (Figure 4) Observing the structure of autophagy vacuoles with a high-power (×5000) electron microscope. Autophagic vacuoles were observed in I/R group (Figures 5). Electron microscopy results show a decrease in autophagy vesicles and an increase in apoptosis after 3-MA pretreatment (Figures 5 (a, b, d), white arrows). The autophagic vacuoles in I/R Rap group is higher (Figure 5).
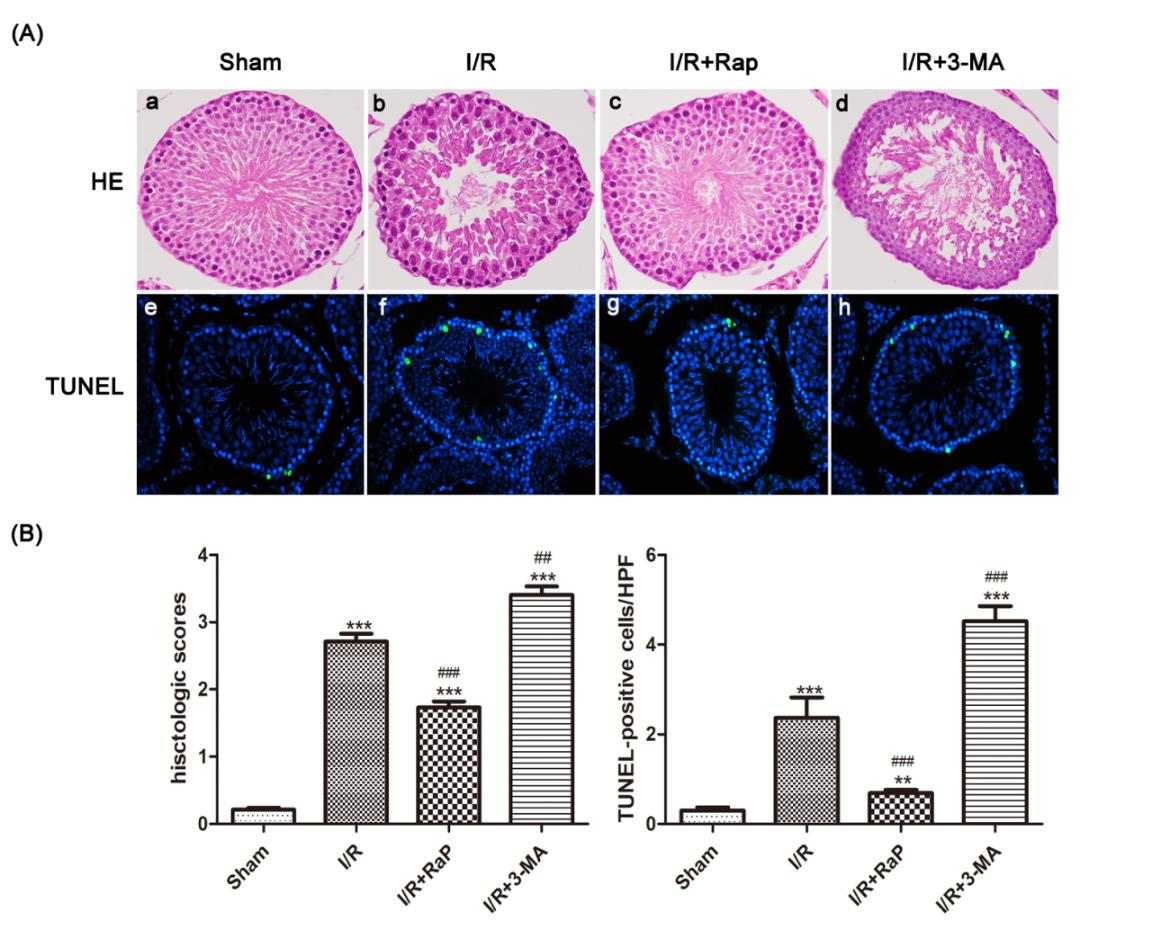 Figure 1. Rat Testicular H-E and TUNEL staining. Representative images of testicular histology with HE staining (original magnification×400) (A(a) to (d)). The Sham group showed no obvious morphological changes(A(a)), (b): significant damage of spermatogenic function, such as extensive seminiferous epithelium injur, the appearance of vacuoles, and a disorderly distribution of spermatogenic cells. (c)There were fewer spermatogenic cells and seminiferous epithelium changes than in the I/R group, (d)Testicular tissue damage was most severe in this group compared to all groups. Nuclei of TUNEL-positive cells are stained green (original magnification×400) (A(e) to (h)). (B) Data were shown as the mean±SD. *P<0.05, **P<0.01, ***P<0.001 versus Sham group; #P<0.05, ##P<0.01, ###P<0.001 versus I/R group. HE: hematoxylin-eosin; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
Figure 1. Rat Testicular H-E and TUNEL staining. Representative images of testicular histology with HE staining (original magnification×400) (A(a) to (d)). The Sham group showed no obvious morphological changes(A(a)), (b): significant damage of spermatogenic function, such as extensive seminiferous epithelium injur, the appearance of vacuoles, and a disorderly distribution of spermatogenic cells. (c)There were fewer spermatogenic cells and seminiferous epithelium changes than in the I/R group, (d)Testicular tissue damage was most severe in this group compared to all groups. Nuclei of TUNEL-positive cells are stained green (original magnification×400) (A(e) to (h)). (B) Data were shown as the mean±SD. *P<0.05, **P<0.01, ***P<0.001 versus Sham group; #P<0.05, ##P<0.01, ###P<0.001 versus I/R group. HE: hematoxylin-eosin; TUNEL: terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling; I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
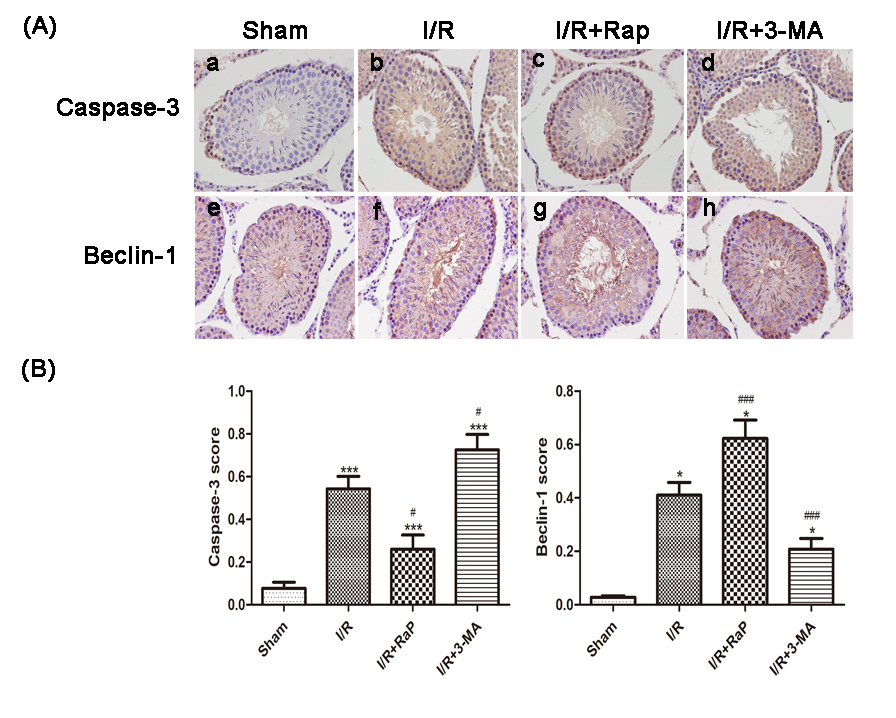 Figure 2. Representative of immunoblotting images of caspase-3 (A(a) to (d)) and Beclin-1 (A(e) to (h)) (original magnification×400). Quantification of the percentage of caspase-3 expression and Beclin-1 expression of all groups (B). Compared with the I/R group, the caspase-3 score was higher, but the Beclin-1 score was lower when pretreated with 3-MA. The caspase-3 score was lower, but Beclin-1 score was higher when pretreated with rapamycin. Data were shown as the mean±SD. *P<0.05, ***P<0.001 versus Sham group; #P<0.05, ###P<0.001 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
Figure 2. Representative of immunoblotting images of caspase-3 (A(a) to (d)) and Beclin-1 (A(e) to (h)) (original magnification×400). Quantification of the percentage of caspase-3 expression and Beclin-1 expression of all groups (B). Compared with the I/R group, the caspase-3 score was higher, but the Beclin-1 score was lower when pretreated with 3-MA. The caspase-3 score was lower, but Beclin-1 score was higher when pretreated with rapamycin. Data were shown as the mean±SD. *P<0.05, ***P<0.001 versus Sham group; #P<0.05, ###P<0.001 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
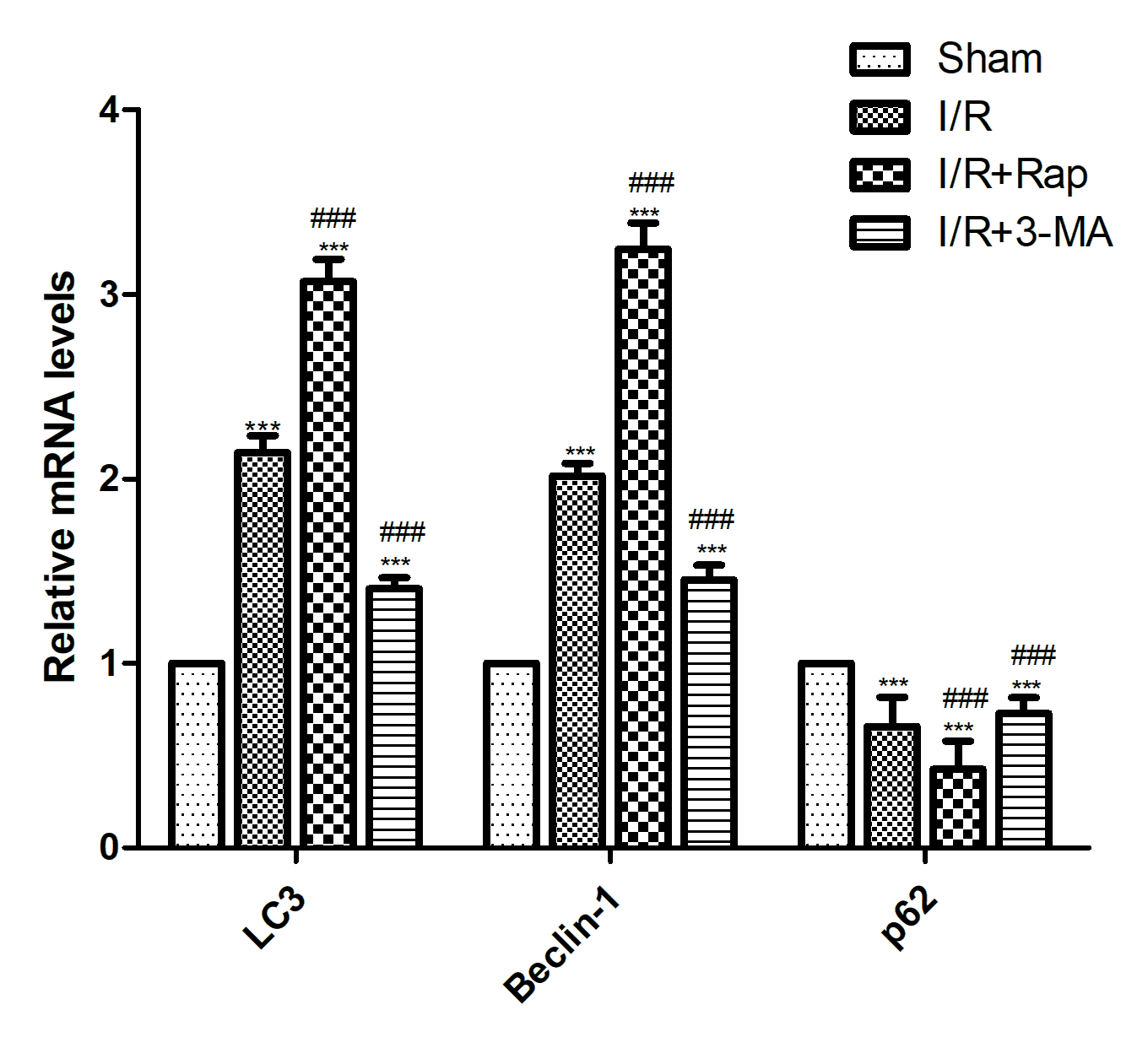 Figure 3. Expression of mRNA levels of LC3, Beclin-1, and p62 detected by qRT-PCR. The mRNA expressions of LC3 and Beclin-1 were increased but p62 was decreased after I/R injury. Compared with the I/R group, 3-MA pretreatment showed higher levels of p62 but lower levels of LC3 and Beclin-1, while rapamycin pretreatment showed higher levels of LC3 and Beclin-1 and lower level of p62. Data were shown as the mean±SD. ***P<0.001 versus Sham group; ###P<0.001 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
Figure 3. Expression of mRNA levels of LC3, Beclin-1, and p62 detected by qRT-PCR. The mRNA expressions of LC3 and Beclin-1 were increased but p62 was decreased after I/R injury. Compared with the I/R group, 3-MA pretreatment showed higher levels of p62 but lower levels of LC3 and Beclin-1, while rapamycin pretreatment showed higher levels of LC3 and Beclin-1 and lower level of p62. Data were shown as the mean±SD. ***P<0.001 versus Sham group; ###P<0.001 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
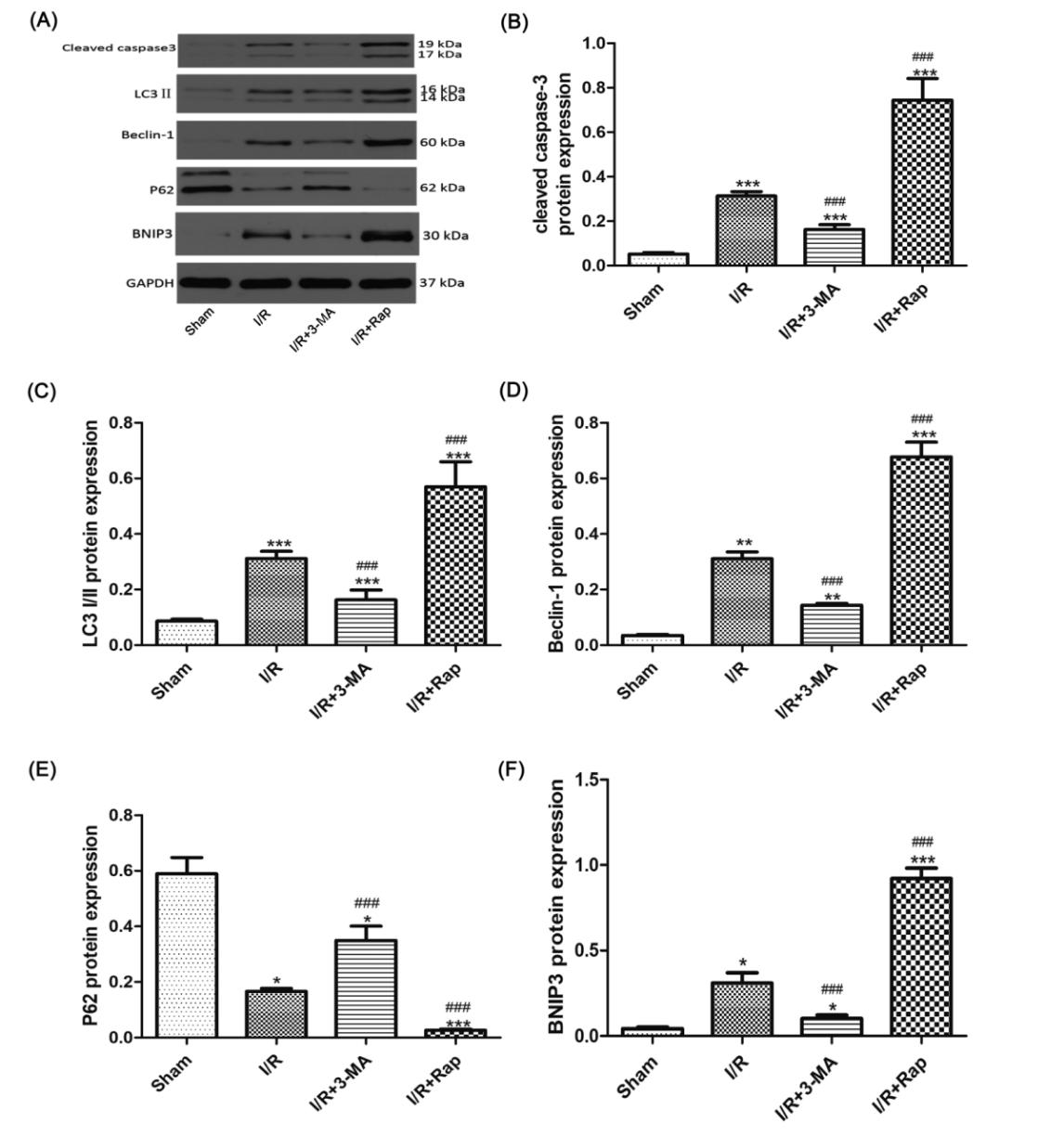 Figure 4. Expression of protein levels of LC3, Beclin-1, and p62 detected by Western blot. Representative blots of caspase-3, LC3 I/II, Beclin-1, and P62, BNIP3, GAPDH was used as a loading control (A). The relative densities of the bands in each lane were analyzed and normalized to GAPDH (B to F). The data were expressed as a percentage of the Sham group, with higher protein levels of p62 but lower of LC3, BNIP3, and Beclin-1 in the I/R+3-MA group, and higher protein levels of LC3, BNIP3 and Beclin-1 and lower of p62 in the I/R+Rap group. Data were shown as the mean±SD. *P<0.05, **P<0.01, ***P<0.01 versus Sham group, ##P<0.01, ###P<0.001 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
Figure 4. Expression of protein levels of LC3, Beclin-1, and p62 detected by Western blot. Representative blots of caspase-3, LC3 I/II, Beclin-1, and P62, BNIP3, GAPDH was used as a loading control (A). The relative densities of the bands in each lane were analyzed and normalized to GAPDH (B to F). The data were expressed as a percentage of the Sham group, with higher protein levels of p62 but lower of LC3, BNIP3, and Beclin-1 in the I/R+3-MA group, and higher protein levels of LC3, BNIP3 and Beclin-1 and lower of p62 in the I/R+Rap group. Data were shown as the mean±SD. *P<0.05, **P<0.01, ***P<0.01 versus Sham group, ##P<0.01, ###P<0.001 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
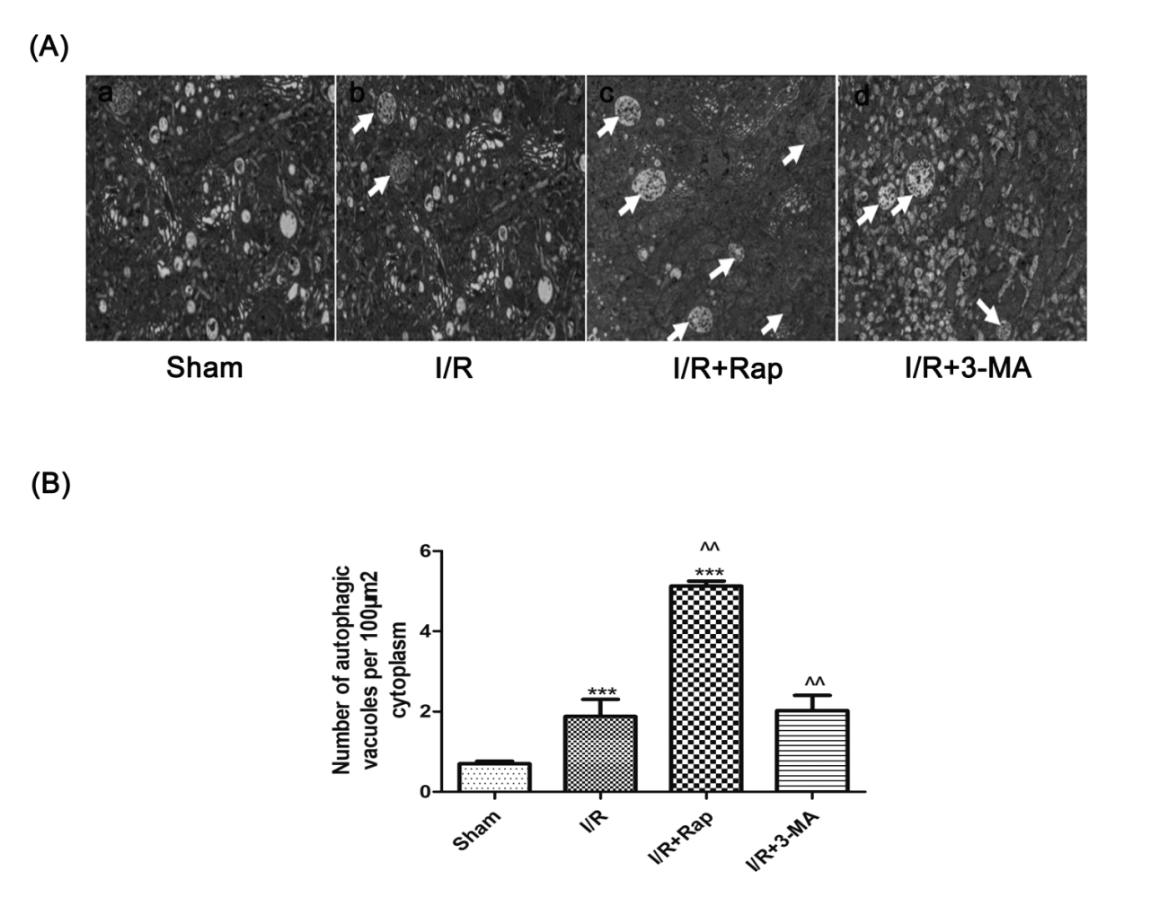 Figure 5. Ultrastructural changes in testicular cells after I/R injury. Representation of high magnification of electron micrographs showing ultrastructural changes structures (A). The Sham group showing healthy nuclei, endoplasmic reticulum, mitochondria, lysosomes, and cytoplasmic Golgi complexes (A(a)). Autophagic vacuoles containing whorls of membranous material and some cytoplasm (white chevrons) were found in the I/R group (A(b)). Apoptosis with disrupted cell membranes displaying cell shrinkage, nuclear and chromatin condensation or aggregation at the edge of the nucleus in the I/R+3-MA group (white arrows) (A(c)). More multiple double- or multiple-membrane autophagic vacuoles containing cytoplasm or undigested organelles were identified in the I/R+Rap group (white arrows) (A(d)). Quantification of the number of autophagic vacuoles per 100 mm2 cytoplasm (B). Data were shown as the mean±SD. ***P<0.001 versus Shamgroup; ^^P<0.01 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
Figure 5. Ultrastructural changes in testicular cells after I/R injury. Representation of high magnification of electron micrographs showing ultrastructural changes structures (A). The Sham group showing healthy nuclei, endoplasmic reticulum, mitochondria, lysosomes, and cytoplasmic Golgi complexes (A(a)). Autophagic vacuoles containing whorls of membranous material and some cytoplasm (white chevrons) were found in the I/R group (A(b)). Apoptosis with disrupted cell membranes displaying cell shrinkage, nuclear and chromatin condensation or aggregation at the edge of the nucleus in the I/R+3-MA group (white arrows) (A(c)). More multiple double- or multiple-membrane autophagic vacuoles containing cytoplasm or undigested organelles were identified in the I/R+Rap group (white arrows) (A(d)). Quantification of the number of autophagic vacuoles per 100 mm2 cytoplasm (B). Data were shown as the mean±SD. ***P<0.001 versus Shamgroup; ^^P<0.01 versus I/R group. I/R: ischemia reperfusion; 3-MA: 3-methyladenine; Rap: Rapamycin.
Our study successfully established an I/R injury model in rats to investigate the relationship between I/R injury and autophagy. This study found that autophagy was activated in testicular tissue after I/R injury. Inhibition of autophagy by 3-MA led to the aggravation of testicular tissue injury, while activation of autophagy by rapamycin was important in the testicular group. Autophagy promotes cell survival or leads to cell death by stimulating various factors [6-8, 20]. The basic level of autophagy is a self-feeding cellular process that degrades cytoplasmic proteins and subcellular organelles in lysosomes, recovers cytoplasmic components, regenerates cell components and energy, and maintains cell and tissue homeostasis [25, 26]. Autophagy is an evolutionarily conserved catabolic process that degrades misfolded or aggregated proteins. The results showed that testicular I/R injury activated autophagy. In this process, the formation of bilayer structures (autophagy) engulfs and transports cellular contents to lysosomes for degradation. LC3 and Beclin-1 proteins are autophagic markers. Compared with the sham operation, rapamycin or 3-MA can enhance or inhibit this activation, respectively, as demonstrated by autophagy-related protein expression. Autophagy-associated proteins increase with renal I/R injury [24 ], LC3-I is converted to LC3-II under phosphatidylethanolamine and participates in autophagy cell formation and promotes their fusion with lysosomes [27]. Beclin-1 is important for autophagy formation and autophagy and lysosome fusion [28]. Similarly, p62 (SQSTM1 or A170) is one of the key substrates of autophagy used to evaluate autophagy flux in some cases. This protein selectively enters the autophagosome by binding to LC3 and is efficiently degraded by autophagy. In addition, autophagic protein p62 participates in autophagy and is degraded, which negatively correlates with autophagic activity [10]. Expression of LC3-II and Belin-1 increased, and expression of p62 decreased in the I/R+Rap group, indicating that rapamycin activated autophagy, whereas the expression of LC3-II and Beclin-1 decreased and p62 increased, indicating that 3-MA inhibited autophagy. LC3 and Beclin-1 had highest expression, p62 had the lowest expression, and bilayer or multi-membrane autophagic vacuoles increased, suggesting that rapamycin activated autophagy. The results of electron microscopy also confirm this relationship. Autophagy's role in I/R injury has attracted more and more attention, but induced autophagy may also have two sides [10].
Studies have reported that increased autophagy activity can help reduce the inflammatory response caused by intestinal I/R29. Tubular injury and cell damage apoptosis are often associated with renal I/R injury [30]. Spermatogenic cells apoptosis is one of the important factors leading to infertility after testicular I/R injury. Apoptosis is often referred to as programmed cell death. Studies indicated that caspase-3 is positively correlated with cell apoptosis. Caspase-3 is a caspase effector, which is important in the process of apoptosis [31]. Compared with the I/R group, the pathological score of testicular injury in the I/R+Rap group was also lower. With decreased expression of TUNEL-positive cells and decreased caspase-3 in this group, rapamycin can effectively inhibit apoptosis of testicular cells. In conclusion, the results show that autophagy is important for cell survival in I/R injury. Of course, this study still has some shortcomings. In this study, only the indicators related to autophagy were detected in this study, but the mechanim of the protective effect of I/R damage was not studied in depth. In future studies, investigating the mechanism of the protective effect of I/R damage will help the development and application of related therapeutic drugs.
None.
Ethics approval
This study has been approved by the Experimental Animal Ethics Committee of the Third Hospital of Wuhan City (SY2019-027).
Data availability
The Data will be available upon request.
Funding
This study was supported by Key Project of General Program of Wuhan Municipal Health Commission (WX20A16).
Authors’ contribution
Hu Z and Zhang W designed the study. Cheng Q and Xu L analyzed the data and drafted the manuscript. Chen YY, Ning JZ and Cheng F revised the original manuscript. All the authors approved the final manuscript.
Competing interests
All authors declare that there is no conflict of interest in the publication of this study.
- Payabvash S, Salmasi AH, Kiumehr S, Tavangar SM, Nourbakhsh B, Faghihi SH, Dehpour AR: Salutary effects of N-acetylcysteine on apoptotic damage in a rat model of testicular torsion. Urol Int 2007, 79(3): 248-254.
- Vaos G, Zavras N: Antioxidants in experimental ischemia-reperfusion injury of the testis: Where are we heading towards. World J Methodol 2017, 7(2): 37-45.
- Zhang S, Zeng Y, Qu J, Luo Y, Wang X, Li W: Endogenous EGF maintains Sertoli germ cell anchoring junction integrity and is required for early recovery from acute testicular ischemia/reperfusion injury. Reproduction 2013, 145(2): 177-189.
- Karaguzel E, Kadihasanoglu M, Kutlu O: Mechanisms of testicular torsion and potential protective agents. Nat Rev Urol 2014, 11: 391-399.
- Hou Y, Wang J, Feng J: The neuroprotective effects of curcumin are associated with the regulation of the reciprocal function between autophagy and HIF-1α in cerebral ischemia-reperfusion injury. Drug Des Devel Ther 2019, 13: 1135-1144.
- Uchiyama Y: Autophagic cell death and its execution by lysosomal cathepsins. Arch Histol Cytol 2001, 64(3): 233-246.
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z: Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 2007, 26(7): 1749-1760.
- Bursch W: The autophagosomal-lysosomal compartment in programmed cell death. Cell Death Differ 2001, 8(6): 569-581.
- Lenoir O, Tharaux PL, Huber TB: Autophagy in kidney disease and aging: lessons from rodent models. Kidney Int 2016, 90(5): 950-964.
- Zhang YL, Zhang J, Cui LY, Yang S: Autophagy activation attenuates renal ischemia-reperfusion injury in rats. Exp Biol Med(Maywood) 2015, 240(12): 1590-1598.
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T: LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 2000, 19(21): 5720-5728.
- Mizushima N, Yoshimori T, Levine B: Methods in mammalian autophagy research. Cell 2010, 140(3): 313-326.
- Bjørkøy G, Lamark T, Johansen T: p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy 2006, 2(2): 138-139.
- Kroemer G, Mariño G, Levine B: Autophagy and the integrated stress response. Mol Cell 2010, 40(2): 280-293.
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G: Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 2007, 8(9): 741-752.
- Cai Z, Yan LJ: Rapamycin, Autophagy, and Alzheimer's Disease. J Biochem and Pharmacol Res 2013, 1(2): 84-90.
- Klionsky DJ, Cuervo AM, Seglen PO: Methods for monitoring autophagy from yeast to human. Autophagy 2007, 3(3): 181-206.
- Greenstein A, Schreiber L, Matzkin H: The effect of methylene blue on histological damage after spermatic cord torsion in a rat model. BJU Int 2001, 88(1): 90-92.
- Turkmen S, Mentese A, Karaguzel E, Karaca Y, Kucuk A, Uzun A, Yulug E, Turedi S: A comparison of the effects of N-acetylcysteine and ethyl pyruvate on experimental testicular ischemia-reperfusion injury. Fertil Steril 2012, 98(3): 626-631.
- Quintaes IP, Tatsuo ES, Paulo DN, Musso C, Boasquevisque PC: Decompressive fasciotomy in testicular torsion of the spermatic cord in rats. Acta Cir Bras 2013, 28(6): 423-439.
- Dokmeci D, Kanter M, Inan M, Aydogdu N, Basaran UN, Yalcin O, Turan FN: Protective effects of ibuprofen on testicular torsion/detorsion-induced ischemia/reperfusion injury in rats. Arch Toxicol 2007, 81(9): 655-663.
- Amirhassani S, Mehrabi S, Hosseinipanah SM, Iloon Kashkouli A, Torabian S, Moslemi MK: Does intraperitoneal injection of propofol prior to detorsion improve testes weight and histopathological findings in a rat model. Res Rep Urol 2017, 9: 101-105.
- Chien CT, Shyue SK, Lai MK: Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation 2007, 84(9): 1183-1190.
- Price PM, Safirstein RL, Megyesi J: The cell cycle and acute kidney injury. Kidney Int 2009, 76(6): 604-613.
- Finn PF, Dice JF: Proteolytic and lipolytic responses to starvation. Nutrition 2006, 22(7-8): 830-844.
- Cuervo AM: Autophagy: many paths to the same end. Mol Cell Biochem 2004, 263(1): 55-72.
- Lo S, Yuan SS, Hsu C, Cheng YJ, Chang YF, Hsueh HW, Lee PH, Hsieh YC: Lc3 over-expression improves survival and attenuates lung injury through increasing autophagosomal clearance in septic mice. Ann Surg 2013, 257(2): 352-363.
- Zhang LX, Zhao HJ, Sun DL, Gao SL, Zhang HM, Ding XG: Niclosamide attenuates inflammatory cytokines via the autophagy pathway leading to improved outcomes in renal ischemia/reperfusion injury. Mol Med Rep 2017, 16(2): 1810-1816.
- Li Z, Wang G, Feng D, Zu G, Li Y, Shi X, Zhao Y, Jing H, Ning S, Le W: Targeting the miR-665-3p-ATG4B-autophagy axis relieves inflammation and apoptosis in intestinal ischemia/reperfusion. Cell Death Dis 2018, 9(5): 483.
- Lee HT, Park SW, Kim M, Ham A, Anderson LJ, Brown KM, D'Agati VD, Cox GN: Interleukin-11 protects against renal ischemia and reperfusion injury. Am J Physiol Renal Physiol 2012, 303(8): F1216-1224.
- Lakhani SA, Masud A, Kuida K, Porter GA Jr, Booth CJ, Mehal WZ, Inayat I, Flavell RA: Caspases 3 and 7: key mediators of mitochondrial events of apoptosis. Science 2006, 311(5762): 847-851.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript