Research Article | Open Access
Comparative phytochemical analysis of aqueous leaf extracts of locally grown and imported variants of Nicotiana Tabacum in Southeastern Nigeria
Nelson I. Oguanobi1, 2, Chioli P. Chijioke1, 2, Samuel I. Ghasi1, Nkoyo I. Nubila1, Kenneth I. Nwadike1, Obinna C. Nwoke1, Caleb C. Okolo1, 2
1Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, College of Medicine, University of Nigeria, Enugu Campus, Nigeria.
2Department of Medicine, University of Nigeria Teaching Hospital, Enugu, Nigeria.
Correspondence: Nelson I. Oguanobi (Department of Pharmacology and Therapeutics, Faculty of Basic Clinical Sciences, College of Medicine, University of Nigeria, Enugu Campus, Nigeria; Email: nelson.oguanobi@unn.edu.ng).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2025, 5: 74-80. https://doi.org/10.32948/ajpt.2025.11.20
Received: 13 Dec 2025 | Accepted: 24 Dec 2025 | Published online: 30 Dec 2025
Methods The study adopted an experimental research design consisting of laboratory assays of tobacco products marketed in Enugu metropolis, Southeast Nigeria. Two major sources of tobacco were identified during a preliminary survey of tobacco markets in Enugu metropolis. These were A, locally-grown tobacco obtained from Southwestern Nigeria and B, foreign-grown and processed tobacco leaves. Dry leaves of tobacco purchased from the two sources were analyzed for compositions, qualitative and quantitative phytochemistical contents.
Results The local Nicotiana tabaccum leaf extract had higher absolute and relative concentration of Anthracyanin (10.18±1.79mg/kg; versus 4.99±0.75mg/kg), Cyanogenic glycoside (7.88±1.64mg/kg; 4.86±0.43mg/kg), Rutin (11.92±1.65; 4.98±0.87mg/kg), and Flavonones (7.01±0.76mg/kg; 3.55±0.41mg/kg). On the other hand, the foreign tobacco was found to have greater content of Cardiac glycoside, Steroids, Epicatechin, Phytate and Oxalate (7.93±0.59; 4.26±0.88mg/kg, 10.99±1.85; 6.11±0.38mg/kg, 21.00±2.18; 9.82±1.20mg/kg, 8.98±1.50; 4.40±0.54mg/kg, and 23.49±2.58; 12.94±1.31mg/kg, respectively for foreign and local tobacco leaf extracts; p=0.0001). Some components were however, exclusively specie specific for the foreign tobacco (Lunamarin, Naringin, Flavon-3-ol, Naringenin) and the local tobacco (Proanthocyanin, Tanin, Flavone, Resveratrol), being virtually undetected in the other species. Oxalates are the most abundant in both foreign and local extracts (17.06%; 10.77%), followed in decreasing order by Epicatechin (15.30%; 8.19%) and Sapogenin (9.93%; 8.34%).
Conclusion The observed significantly high abundance of phytochemical constituents in the Nigerian locally-grown Nicotiana tabacum leaf extract in this study underscores the potentially rich medicinal, industrial and economic value of this plant and the need for improved processing and handling techniques to eliminate the potential risk associated with the observed high levels of cyanogenic glycosides.
Key words comparative, phytochemical, analysis, extracts, nicotiana tabacum, nigeria
Nicotiana tabacum is endowed with numerous primary and secondary bioactive metabolites which play essential roles in their adaptation to the environments, and their synthesis is often induced by different kinds of biotic and/or abiotic stresses [2]. The concentrations of phytochemical constituents in a plant are affected by environmental factors, cultivation methods and diseases [3].
It is also known that the types and concentrations of phytochemicals in tobacco leaves change during ripening, drying, fermentation, and storage period, and are influenced by a variety of factors such as processing methods, climate and temperature [4, 5, 6].
Hence, we postulate that substantial variation in phytochemical constituents may exist between foreign and local grown varieties of Nicotiana tabacum marketed in Southeastern Nigeria.
The specific objective of the study was to analyze and compare the phytochemical composition of dried leaf extracts of foreign and Nigerian locally grown varieties of Nicotiana tabacum.
Two major sources of tobacco were identified during a preliminary survey of tobacco markets in Enugu metropolis. These were: A, Locally grown tobacco; and B, Foreign grown and processed tobacco leaf variety. The German Virginia tobacco (FCV German Leaf GmbH & Co. KG), which was selected for the study was among the more commonly used foreign tobacco. Repesentative single batches samples of local and foreign varieties of dry tobacco leaves purchased from a major tobacco markets in Enugu metropolis were appropriately labeled for identification. The leaf samples were botanically authenticated at the Department of Botany University of Nigeria Nsukka, Nigeria. All the samples were analyzed for compositions, qualitative and quantitative phyto-chemistry.
Preparation of extracts
Dried leaf extracts of Nicotian tabacum were ground into coarse powder. The coarse powder was subjected to aqueous extraction in a soxhlet apparatus for 10 hours as follows: Fifty grams of the powdered leaves were placed in the inner thimble of the soxhlet extractor apparatus. Water was passed through the inner timble via a condenser with reflux system, from a round bottom flask containing 450ml of distilled water and placed on a thermostatic heating mantle regulated at 100 ℃.
After filtration through Whatman filter paper No. 40, the filtrate was slowly evaporated to dryness on an electrothermal heating mantle regulated at 60 ℃. The extracts were stored in screwed cap vials at 4-8℃ until further use.
Phytochemical Analysis
Phytochemical analysis was done at Spring Board Research Laboratory Awka, Anambra state, Nigeria. Aqueous extract was evaluated for the presence of alkaloids, tannins, saponins, flavonoids and phenolic compounds using methods described by Zou et al [7], Kelley and Nelson [8].
Quantification by Gas chromatography equipped with a flame ionization detector (GC-FID).
The quantitative analysis of phytochemicals was performed on a BUCK M910 Gas chromatography equipped with a flame ionization detector [9]. A RESTEK 15 meter MXT-1 column (15m x 250um x 0.15um) was used. The injector temperature was 280 ℃ with splitless injection of 2ul of sample and a linear velocity of 30 cms-1, Helium 5.0 pa.s was the carrier gas with a flow rate of 40 mlmin-1. The oven operated initially at 200 ℃, It was heated to 330 ℃ at a rate of 3℃ min-1 and was kept at this temperature for 5min. The detector operated at a temperature of 320 ℃.
The quantification of non-volatile, high-molecular-weight, and thermally unstable compounds (such as Anthocyanins, Rutin, Tannins, Cardiac Glycosides, Cyanogenic Glycosides) was done using Gas Chromatography linked with a flame ionization detector (GC-FID) after applying a chemical derivatization process that converted them into more volatile, thermally stable, and easily detectable forms [9].
Phytochemicals were determined by the ratio between the area and mass of internal standard and the area of the identified phytochemicals. The concentration of the different phytochemicals was expressed in mg/kg.
Statistical analysis
Data were presented as means ± standard deviation for continuous variables and as proportions for categorical variables. Comparison of continuous variables between the foreign and local tobacco products were made with independent Student’s t-test. For discrete variables distribution between groups were compared with Chi- square test and Fishers exact test as appropriate (where an expected cell is less than 5). All statistical analyses were carried out using the Statistical Packages for Social Sciences (SPSS Inc. Chicago Illinois) software version 25.0. Statistical tests with probability values less than 0.05 were considered statistically significant.
The spatial distribution of the various phytochemical components on chronographic column of the Gas chromatography equipped with a flame ionization detector (GC-FID) used for their quantification which is determined by the retention time, are displayed in Plates 1 and 2 (Figure 2 to 3).
|
Table 1. Comparison of absolute concentration of phytochemicals in foreign and local tobacco extracts: A quantitative analysis. |
||||
|
Components |
Foreign tobacco mg/kg (SD) |
Local tobacco mg/kg (SD) |
T-test |
Significance |
|
Proanthocyanin |
ND |
8.49 (1.67) |
- |
- |
|
Lunamarin |
5.26(0.13) |
ND |
- |
- |
|
Naringin |
3.69(0.94) |
ND |
- |
- |
|
Cardiac glycoside |
7.93(0.59) |
4.26(0.88) |
8.5178 |
0.0001* |
|
Anthocyanin |
4.99 (0.75) |
10.18 (1.79) |
6.5553 |
0.0001* |
|
Flavon 3 ol |
5.93 (0.88) |
ND |
- |
- |
|
Ribalinidine |
2.75 (0.66) |
1.86 (0.25) |
3.1316 |
0.0106* |
|
Naringenin |
7.18 (0.95) |
ND |
- |
- |
|
Sparteine |
6.11 (0.71) |
6.61 (0.87) |
1.0713 |
0.3091 |
|
Cyanogenic glycoside |
4.86 (0.43) |
7.88 (1.64) |
4.3747 |
0.0014* |
|
Rutin |
4.98 (0.87) |
11.92 (1.65) |
9.1286 |
0.0001* |
|
Flavonones |
3.55 (0.41) |
7.01 (0.76) |
9.8085 |
0.0001* |
|
Steroids |
10.99(1.85) |
6.11 (0.38) |
6.3306 |
0.0001* |
|
Kaempferol |
3.38(0.49) |
4.69 (0.35) |
5.3788 |
0.00031* |
|
Epicatechin |
21.00 (2.18) |
9.82(1.20) |
11.0270 |
0.0001* |
|
Phytate |
8.98 (1.50) |
4.40 (0.54) |
7.030 |
0.0001* |
|
Oxalate |
23.49 (2.58) |
12.94 (1.31) |
8.8974 |
0.0001* |
|
Catechin |
1.35 (0.12) |
1.13(0.37) |
1.3689 |
0.201 |
|
Sapogenin |
10.89 (1.18) |
10.02 (1.27) |
1.2285 |
0.2474 |
|
Proanthcyanin |
ND |
8.49(1.67) |
- |
- |
|
Tannin |
ND |
3.00 (0.73) |
- |
- |
|
Flavone |
ND |
8.49 (0.76) |
- |
- |
|
Resveratrol |
ND |
1.29 (0.73) |
- |
- |
|
*Statistically significant (independent T-test), ND= Not detectable. |
||||
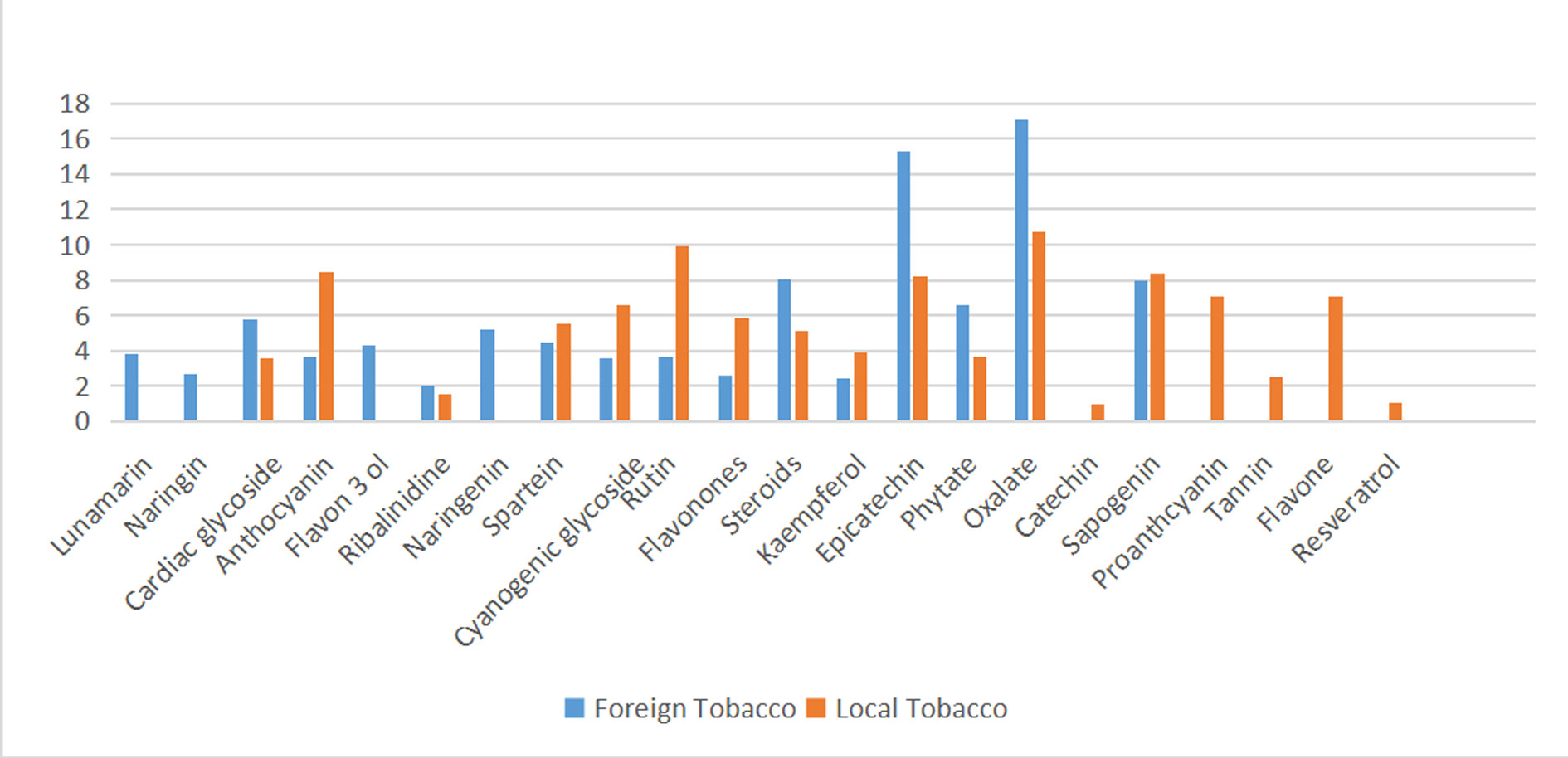 Figure 1. Percentage(relative) phytochemical composition of foreign and local tobacco extracts.
Figure 1. Percentage(relative) phytochemical composition of foreign and local tobacco extracts.
 Figure 2. Plate 1-Column chromatography showing quantitative phytochemical analysis for aqueous foreign.
Figure 2. Plate 1-Column chromatography showing quantitative phytochemical analysis for aqueous foreign.
 Figure 3. Plate 2-Column chromatography showing Quantitative phytochemical analysis for aqueous local tobacco leaf extract.
Figure 3. Plate 2-Column chromatography showing Quantitative phytochemical analysis for aqueous local tobacco leaf extract.
Phytochemicals can be classified into major categories, such as alkaloids, carotenoids and polyphenols. Polyphenol compounds constitute a diverse group which include simple phenols, flavonoids, and non-flavonoids. Coumarins and Phenolic acids are sub-groups of simple phenols which also includes phytochemical sub-classes such as hydroxybenzoic acids, hydroxycinnamic acids, flavonols, flavones, iso-flavones, flavanols, flavanones, anthocyanins, chalcones. Flavanols are further classified as catechins, epicatechins, and proanthocyanidins [11, 12]. Tannins, lignans, and stilbenes are classified as non-flavonoids [11, 12].
Nicotiana tabacum is endowed with numerous bioactive metabolites which account for the rich phytochemical constituents identified in the tobacco leaf extracts in this study. A total of 22 phytochemicals were identified in tobacco extracts evaluated in this study. Major constituents found include; Oxalates, Epicatechin, Anthrocyanin, Rutin, Sapogenins, Steroids and glycosides (cardiac and cyanogenic). Similar findings were reported by several studies within and outside Nigeria [13-16]. The abundance of phytoactive constituent such as Sapogenin, anthracyanin and Rutin is responsible for the medicinal properties of Nicotiana tabacum. However, some adverse effects of the plant have been noted and attributable to the presence of toxic alkaloids [17].
A comparative phytochemical analysis of crude leaf extracts of locally grown and imported varieties of Nicotiana tabacum showed significant variations in concentration and types of constituents. The local Nicotiana tabacum leaf extract has higher abundance of Anthracyanin, Cyanogenic glycoside, Rutin, and Flavonones. These Poly-Phenolic phytochemicals are plant components with poly-hydroxyl groups, which are widely distributed in plants tissue and commonly used as antioxidants in the human diet due to their high reactive oxygen radical scavenging capacity [18, 19].
On the other hand, the foreign tobacco was found to have greater content of Cardiac glycoside, Steroids, Epicatechin, Phytate and Oxalate. It is known that steroid hormones are produced in mammalian female and male gonads, and adrenal glands. However, reports of steroid production by plants are also been noted in several studies [20, 21]. Steroids present in the plant help its defense mechanism against certain viruses and pathogens. Previous studies have found that tobacco plants are capable of synthesizing animal steroid hormone such as progesterone and androst-4-ene-3, 17-dione, testosterone, and estradiol [20-22].
Some phytochemical components were however, exclusively specie specific for the foreign tobacco (Lunamarin, Naringin, Flavon-3-ol, Naringenin) and the local tobacco (Proanthcyanin, Tanin, Flavone, Resveratrol), and were virtually undetected in the other species. Tobacco leaves have been found to contain many kinds of polyphenols, among which phenolic acid and flavonoid glycosides constitute a large proportion [23, 24].
Germany is a leading source of imported raw tobacco in Nigeria and the main source of foreign tobacco in Enugu metropolis [25].The obvious differences in geographical growth and post-harvest processing would account for the variation in phytochemistry found in this study between crude extracts of foreign and locally grown tobacco.
The concentrations of phytochemical constituents in a plant are affected by the environmental stresses such as drought, heat/cold, mineral deficiencies and diseases. They play essential roles in the adaptation of plants to their environments, and their synthesis is often induced by different kinds of stress conditions [1-3].
Several studies have shown that abiotic stress such as drought and salinity stress enhanced the total phenolic content, and flavonoid contents and individual phenolic acids and flavonoid compounds [1, 3]. The intensity of the antioxidant activities of these agents is based on their ability to combine with free radicals and their function is to protect plants from ultraviolet radiation, fungal pathogens, and avoid cells damaged by oxidative stress [26]. In addition, polyphenols also contribute to plant color and aroma attributes [1]. Phenolic compounds identified in our study samples include Flavon- 3- ol, Flavonones, Favones, Tanins, Kaempferols, Rutin, and anthocyanins. These were significantly more abundant in the local tobacco variety.
Saponins and Sparteine, a lupin alkaloid were found to be well distributed in all the extracts evaluated in this study. These phytochemicals are known to be increased in content when plants are subjected to water stress and stress saline conditions and composition may vary markedly depending on the surrounding environmental factors.
The significantly higher levels of cyanogenic glycosides in the local leaf extracts reflect an essential natural chemical defense mechanism of the plant to deter herbivores by releasing cyanide when plant tissues are damaged [27]. However, the high cyanogenic glycoside content in Nigerian tobacco products poses a serious health risk arising from the release of toxic hydrogen cyanide upon processing or smoking, and potentially causing acute poisoning or chronic neurological disorder. There is therefore a need for improved tobacco processing methods to reduce cyanogenic glycosides and lower health risks for tobacco farmers and consumers [27, 28].
Several studies have reported high cyanide levels in various other Nigerian staple foods such as cassava, sorghum, cocoyam, certain seeds/nuts (tiger nuts, cashew nuts) and certain leafy vegetables like bitter leaf [28, 29]. Traditional detoxification methods such as fermentation, drying, and cooking have be employed to reduce cyanide levels in natural food produce to safe limits for consumption [29].
The findings from analysis of the chemical composition of leaf extracts of Nicotiana tabacum evaluated in this study corroborate previous report of variations in the phytochemical composition depending on plant genetics, growing conditions, processing technique and geographical location. The observed significantly high abundance of phytochemical constituents in in the locally grown Nicotiana tabacum leaf extract in this study has far reaching implications especially considering the potential medicinal, industrial and economic benefits which can be harnessed and exploited.
However, the high levels cyanogenic glycosides in Nigerian tobacco leaf extracts may pose significant human health risks, requiring awareness, improved processing techniques, and greater regulatory attention to protect consumers and tobacco workers from cyanide toxicity.
No applicable.
Ethics approval
No applicable.
Data availability
The authors confirm that the data supporting the findings of this research work are available within the article. Any other data/information will also be available from the corresponding author on request.
Funding
None received.
Authors’ contribution
N.I.O was responsible for conceptualization, study design, data analysis and writing of manuscript; C.P.C and S.I.G shared in the study design and data analysis; N.I.N, O.C.N, K.I.N and C.C.O participated in data collection and data analysis. All authors shared in the final preparation and review of the manuscript.
Competing interests
The authors declare that they have no competing interests.
- Sarker U, Oba S: Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J Sci Food Agric 2019, 99(5): 2275-2284.
- Anusuya M, Princy V, Nagaveni A, Suganthi M, Poonkodi K, Jayanthi E: Investigation of Phytochemical Constituents of Tobacco (Nicotiana Tobacum L.) Methanol Extract. Mol Biol 2021, 10: 277-278.
- Sarker U, Oba S: Drought stress effects on growth, ROS markers, compatible solutes, Phenolics, Flavonoids, and Antioxidant activity in Amaranthus tricolor. Appl Biochem Biotechnol 2018, 186(4): 999-1016.
- Banoˇzi´c M, Babi´c J, Joki´c S: Recent advances in extraction of bioactive compounds from tobacco industrial waste-a review. Ind Crops Prod 2020, 144: 112009.
- Bhisey RA: Chemistry and toxicology of smokeless tobacco. Indian J Cancer 2012, 49 (4): 364-372.
- Xiao X, Chen B, Chen Z, Zhu L, Schnoor JL: Insight into multiple and multilevel structures of biochars and their potential environmental applications: a critical review. Environ Sci Technol 2018, 52(9): 5027-5047.
- Zou X, Amrit BK, Abu-Izneid T, Aziz A, Devnath P, Rauf A, Mitra S, Emran TB, Adil AH, Mujawah AAH, et al: Current advances of functional phytochemicals in Nicotiana plant and related potential value of tobacco processing waste: A review. Biomed Pharmacother 2021, 143: 112191-112203.
- Kelly DA, Nelson R: Characterization and quantification by gas chromatography of Phytochemicals. J Braz Chem Soc 2014, 25: 1-6.
- Bajpai VK, Kim NH, Kim K: Chemical derivatization of pharmaceutical samples prior to Gas-Chromatography and Mass-Spectrometry analysis. Bangladesh J Pharmacol 2016, 11: 852-855.
- Novak W K, Haslberger AG: Substantial equivalence of antinutrients and inherent plant toxins in genetically modified foods. Food Chem Toxicol 2000, 38(6): 473-483.
- Zou X, Bk A, Abu-Izneid T, Aziz A, Devnath P, Rauf A, Mitra S, Emran TB, Mujawah AAH, Lorenzo JM, et al: Current advances of functional phytochemicals in Nicotiana plant and related potential value of tobacco processing waste: A review. Biomed Pharmacother 2021, 143: 112191-112204.
- Aarti Rawat, Rakesh Roshan Mali: Phytochemical Properties and Pharmcological Activities of Nicotiana Tabacum: A Review. Indian J Pharm Biol Res 2013, 1(2): 74-82.
- Oyekunle IP, Nwogu US, Orababa OQ, Candidus NC: Phytochemical, Antimicrobial and Proximate Composition of Nicotiana tabacum Leaves Extract. Int J Innovat Sci Res Technol 2019, 4(5): 406-410.
- Prommaban A, Kheawfu K, Chittasupho C, Sirilun S, Hemsuwimon K, Chaiyana W: Phytochemical, Antioxidant, Antihyaluronidase, Antityrosinase, and Antimicrobial Properties of Nicotiana tabacum L. Leaf Extracts. Evid Based Complement Alternat Med 2022, 29: 5761764.
- Zhang W, Pan X, Fu J, Cheng W, Lin H, Zhang W, Huang Z: Phytochemicals derived from Nicotiana tabacum L. plant contribute to pharmaceutical development. Front Pharmacol 2024, 15: 1372456.
- Popova V, Ivanova T, Stoyanova A, Georgiev V, Hristeva T, Nikolova V, Docheva M, Nikolov N, Damianova S: Phytochemicals in leaves and extracts of the variety “Plovdiv 7” of Bulgarian oriental tobacco (Nicotiana tabacum L.). Trends in Phytocheml Res 2018, 2(1): 27-36.
- Kanmani S, Kumar L, Raveen R, Tennyson S, Arivoli S, Jayakumar M: Toxicity of tobacco Nicotiana tabacum Linnaeus (Solanaceae) leaf extracts to the rice weevil Sitophilus oryzae Linnaeus 1763 (Coleoptera: Curculionidae). J Basic Appl Zool 2021, 82(10): 1-12.
- Zou X, Bk A, Abu-Izneid T, Aziz A, Devnath P, Rauf A, Mitra S, Emran TB, Mujawah AAH, Lorenzo JM et al: Current advances of functional phytochemicals in Nicotiana plant and related potential value of tobacco processing waste: A review. Biomed Pharmacother 2021, 143: 112191-112204.
- Abbas M, Saeed F, Anjum FM, Afzaal M, Tufail T, Bashir MS, Ishtiaq A, Hussain S, Suleria HAR: Natural polyphenols: an overview. Int J Food Prop 2017, 20 (8): 1689-1699.
- Zhao L, Zhang H, Hao T, Li S: In vitro antibacterial activities and mechanism of sugar fatty acid esters against five food-related bacteria. Food Chem 2015, 187: 370-377.
- Wang H, Zhao M, Yang B, Jiang Y, Rao G: Identification of polyphenols in tobacco leaf and their antioxidant and antimicrobial activities. Food Chem 2008, 107(4): 1399-1406.
- Malik R, Bokhari TZ, Siddiqui MF, Younis U, Hussain MI, Khan JB: Antimicrobial activity of Nerium oleander L. and Nicotiana tabacum L.: a comparative study. Pak J Bot 2015, 47 (4): 1587-1592.
- Docheva M, Dagnon S, Statkova-Abeghe S: Flavonoid content and radical scavenging potential of extracts prepared from tobacco cultivars and waste. Nat Prod Res 2014, 28 (17): 1328-1334.
- Rodgman A, Perfetti TA: The chemical components of tobacco and tobacco smoke. Taylor & Francis 2009.
- Annual International Trade Statistics by Country (HS). Trend Economy 2022 (11/14).
- Andersen OM, Markham KR: Flavonoids: chemistry, biochemistry and applications. CRC Press 2006.
- Piršelová B, Jakubčinová J: Plant cyanogenic glycosides: from structure to properties and potential applications. Front Plant Sci 2025, 16: 1612132.
- Onojah PK, Odin EM: Cyanogenic Glycoside in Food Plants. Int J Innovat Sci Math 2015, 3(4): 2347-9051.
- Okolie NP, Ugochukwu EN: Cyanide contents of some Nigerian legumes and the effect of simple processing. Food Chem 1989, 32(3): 209-216.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript