Research Article | Open Access
Isolation and quantification of Scopoletin from leaves and marketed formulation of Morinda citrifolia L.
K. Suresh Kumar1, N. Kiruthiga1, R. Arivukkarasu2, S. Dhinesh Kumar1, M. Sureka3
1Department of Pharmaceutical Chemistry, KMCH College of Pharmacy, Coimbatore, Tamilnadu, India.
2Department of Pharmacognosy, KMCH College of Pharmacy, Coimbatore, Tamilnadu, India.
3Department of Pharmaceutical Analysis, KMCH College of Pharmacy, Coimbatore, Tamilnadu, India.
Correspondence: Department of Pharmaceutical Chemistry, KMCH College of Pharmacy, Coimbatore, Tamilnadu, India.
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2024, 4: 18-25. https://doi.org/10.32948/ajpt.2024.05.05
Received: 30 Apr 2024 | Accepted: 05 May 2024 | Published online: 02 Jun 2024
Methods TLC and HPTLC methods were developed for the isolation and quantification of Scopoletin.
Results The ethanol leaf extract of Morinda citrifolia contains 7.4 mg of Scopoletin per 1 g of extract. Noni juices A, B, and C were found to contain 41 mg, 25.7 mg, and 60.93 mg of Scopoletin per 100 ml, respectively. The quantity of Scopoletin in different brands of Noni juices may vary due to changes in temperature, season, and manufacturing processes. Noni juice sample D did not show the presence of Scopoletin, possibly due to its combination with Aloe vera and Garcinia cambogia.
Conclusion The results indicate that the ethanolic extract of Noni leaf and commercial Noni juice products contain the marker compound Scopoletin. Further in vitro and in vivo experiments are needed to explore its mechanism of action and therapeutic properties.
Key words Morinda citrifolia, Noni fruit, Noni leaves, anticancer activity
Cancer is the second leading cause of death worldwide [9], and approximately 35,000 plant species have been approved by the National Cancer Institute (NCI) for their potential anticancer activity [10]. Chemotherapy, the most common treatment for cancer, is associated with various side effects such as pain, sleep disturbances, nausea, vomiting, anxiety, gastrointestinal disorders, insomnia, fatigue, and cognitive impairments [11]. The use of Complementary and Alternative Medicine (CAM), including products from Morinda citrifolia, has been increasing among cancer patients [12]. Several in vitro and in vivo studies have been conducted on the anticancer activity of noni plants [13-16]. Phytoconstituents isolated from the Noni plant, such as Scopoletin [17-19], Damanacanthal [20, 21], Morenone 1, and Morenone 2 [22, 23], have shown to inhibit the growth of cancer cells. Scopoletin, also known as 6-methoxy hydroxycoumarin, is a derivative of phenolic coumarin and a member of phytoalexins. Despite being a rich source of Scopoletin, there is a lack of literature on the quantification of Scopoletin from the leaves and the marketed formulations of Morinda citrifolia L. Thus, the present study aims to isolate and quantify Scopoletin in the leaves and commercial fruit juices of Morinda citrifolia using HPTLC.
Fresh leaf samples of Morinda citrifolia L. (5 kg) were collected from Eden Nursery and Biotech Pvt. Ltd., in Mettupalayam of Coimbatore district, Tamil Nadu, and authenticated by a scientist from the Botanical Survey of India (BSI), Southern Regional Center, Coimbatore (Authentication no: BSI/SRC/5/23/2021/Tech/194).
Procurement of Noni juices
Four different brands of Noni juice were procured from Arya Vaidya Ayurvedic Shop, Coimbatore, Tamil Nadu.
Chemicals and reagents
Standard Scopoletin (AR) was purchased from Sigma Aldrich Pvt. Ltd., Bangalore. Methanol (AR) and ethanol (AR) were purchased from Qualigens Fine Chemical Pvt. Ltd., Mumbai, and were used as solvents for the preparation of standards and samples. Toluene (AR), ethyl acetate (AR), and glacial acetic acid (AR) were purchased from Thermo Fischer Scientific Pvt. Ltd., Mumbai, and used as the mobile phase for HPTLC analysis.
Equipment used
The extracted plant material was subjected to evaporation of the solvent using an Electrical Water Bath (Guna Enterprises, Model: 1870, Chennai). For HPTLC analysis, a CAMAG HPTLC system (Muttenz, Switzerland) equipped with a Linomat V sample applicator was used. Extracts were applied on aluminium-packed TLC plates (20×10 cm) precoated with silica gel 60F254 (Merck Darmstadt, Germany).
Sample preparation
Leaf Sample: The leaf samples were cleaned properly in running tap water and shadow dried. The dried leaf samples were coarsely ground with a grinder, and the powder was passed through a sieve no. 60. The powdered material was stored in a tightly closed container to protect it from atmospheric moisture. 10 g of leaf powder was mixed with 20 ml of ethanol, and the cold maceration extraction process was followed to extract the phytoconstituents. The extract was filtered through Whatman no. 01 filter paper, and the obtained filtrate was evaporated to dryness. 1 g of the dried residue was dissolved in 5 ml of methanol.
Juice Sample: 15 ml of each of the four different brands of noni juice was taken in a beaker. To each beaker, 15 ml of ethanol was added, and the mixture was sonicated for 20-30 minutes. The samples were then cooled to room temperature and filtered using Whatman no. 01 filter paper. The filtrate was evaporated to dryness, and 1 g of the dried residue was dissolved in 5 ml of methanol.
Standard preparation
1 mg of Scopoletin was dissolved in 3 ml of methanol to prepare the standard solution (1000 µg in 3000 µl).
Quantification of Scopoletin using HPTLC
Stationary Phase: Readymade precoated aluminium plates (0.2 mm thickness) containing silica gel 60 F254.
Mobile Phase: Toluene: Ethyl acetate: Glacial acetic acid (7.5: 2.5: 0.1).
The HPTLC investigation was performed using a CAMAG Linomat 5 instrument with WINCATS 1.4.3 application software for scanning. The plates were prewashed with methanol. Samples (8 µl) and standard (8 µl) were spotted on a precoated silica gel plate with a Camag microlitre syringe. The spotted plate was developed in a TLC Twin trough chamber with the respective mobile phase up to 8 cm. After development, the plates were dried at room temperature, and the spots were visualized under UV light at 254 and 366 nm. Densitometric evaluation of the developed plates was done using a Camag scanner III.
Yield of leaf and fruit juice extracts
The yield of leaf and fruit juice extracts of Morinda citrifolia was calculated. For the leaf extract, the yield was 15%, and for the fruit juice extracts, the yields were as follows: Noni Juice A (21%), Noni Juice B (53%), Noni Juice C (30%), and Noni Juice D (15%) are presented in (Table 1).
Quantification of Scopoletin
The ethanolic leaf extract of Morinda citrifolia was analyzed for Scopoletin content using HPTLC. The extract was found to contain 7.4 mg of Scopoletin per 1 g of extract. Subsequently, the Scopoletin content of different commercial Noni juice products (A, B, C, D) was analyzed. Noni juice A was found to contain 41 mg, Noni juice B contained 25.7 mg, and Noni juice C contained 60.93 mg of Scopoletin per 100 ml. However, Scopoletin was not detected in Noni juice D are presented in (Table 2).
Variability in Scopoletin content
The quantification results revealed significant variability in Scopoletin content among the different Noni juice samples. This variability may be attributed to factors such as temperature, season, and manufacturing processes.
Implications for health benefits
The variability in Scopoletin content among different sources of Noni juice may have implications for its potential health benefits. Further studies are warranted to investigate the relationship between Scopoletin content and health effects.
|
Table 1. Percentage Yield of extract. |
||
|
S.No |
Sample |
Yield (%) |
|
1 |
Leaf powder (10g) |
15% |
|
2 |
Noni juice A (15 ml) |
21% |
|
3 |
Noni juice B (15 ml) |
53% |
|
4 |
Noni juice C (15 ml) |
30% |
|
5 |
Noni juice D (15 ml) |
15% |
|
Table 2. Rf values and quantification of Scopoletin in leaves and fruit juices of Morinda citrifolia. |
|||||
|
Sample |
Amount of sample applied (µl) |
Rf value of Peak obtained |
Area of Peak |
Amount of marker present in applied (µl) |
Presence of marker compound in 100 ml of Sample |
|
Leaf extract of Morinda citrifolia |
8 |
0.60 |
8875.96 |
0.72 |
7.4 |
|
Noni juice A |
8 |
0.41 |
796.88 |
0.13 |
41 |
|
Noni juice B |
8 |
0.40 |
3735.23 |
0.20 |
25.7 |
|
Noni juice C |
8 |
0.33 |
935.70 |
0.076 |
60.93 |
|
Noni juice D |
8 |
- |
- |
- |
- |
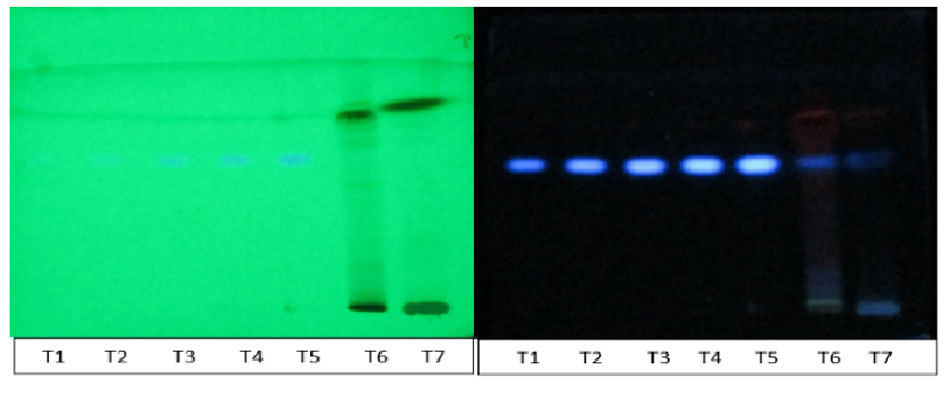 Figure 1. Chromatogram of standard Scopoletin and methanol of leaf Morinda citrifolia (T1-T5→Standard Scopoletin; T6→leaf methanol extract; T7→formulation D).
Figure 1. Chromatogram of standard Scopoletin and methanol of leaf Morinda citrifolia (T1-T5→Standard Scopoletin; T6→leaf methanol extract; T7→formulation D).
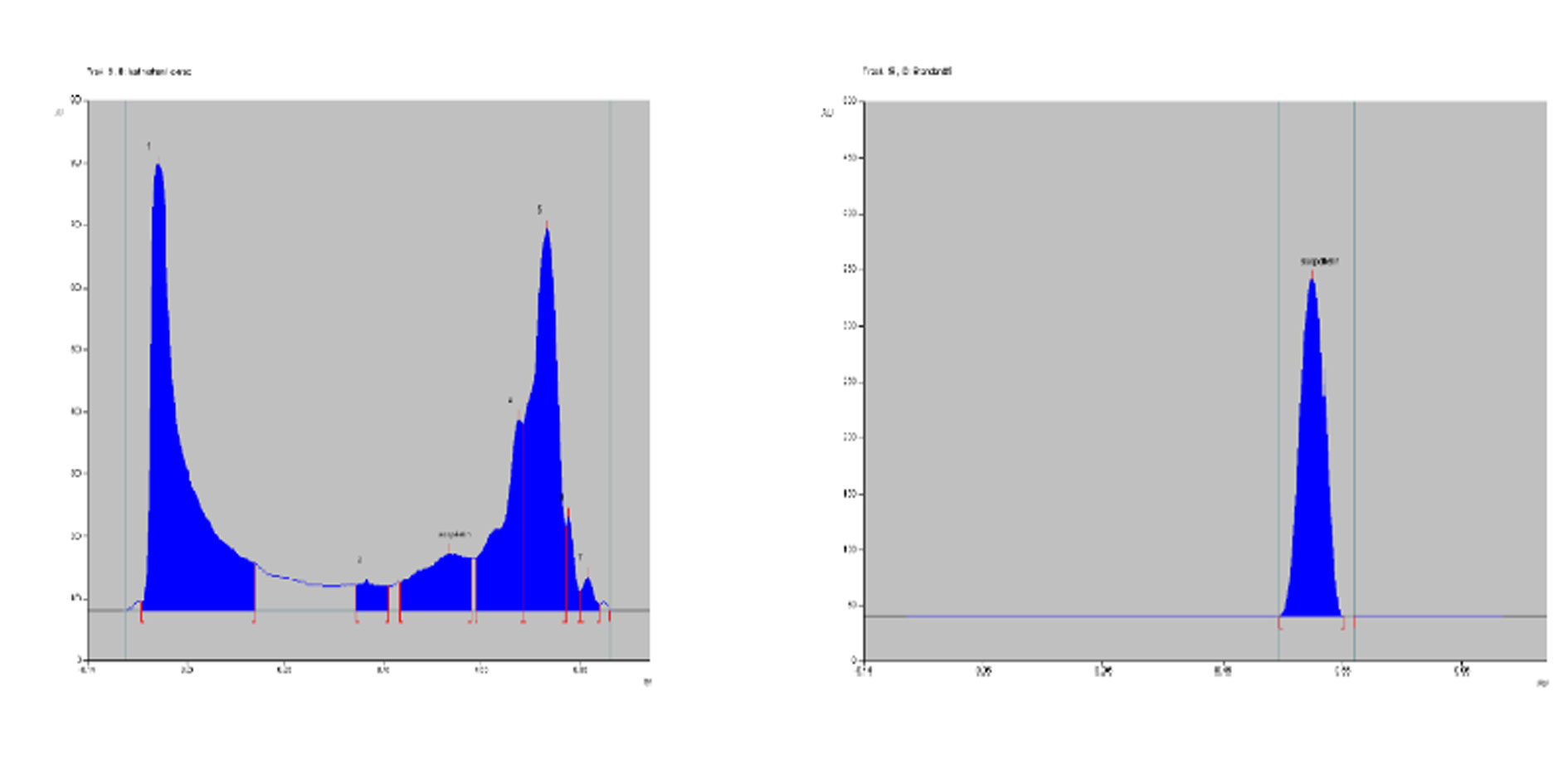 Figure 2. Densitogram of sample leaf methanol extract and standard Scopoletin at 366nm.
Figure 2. Densitogram of sample leaf methanol extract and standard Scopoletin at 366nm.
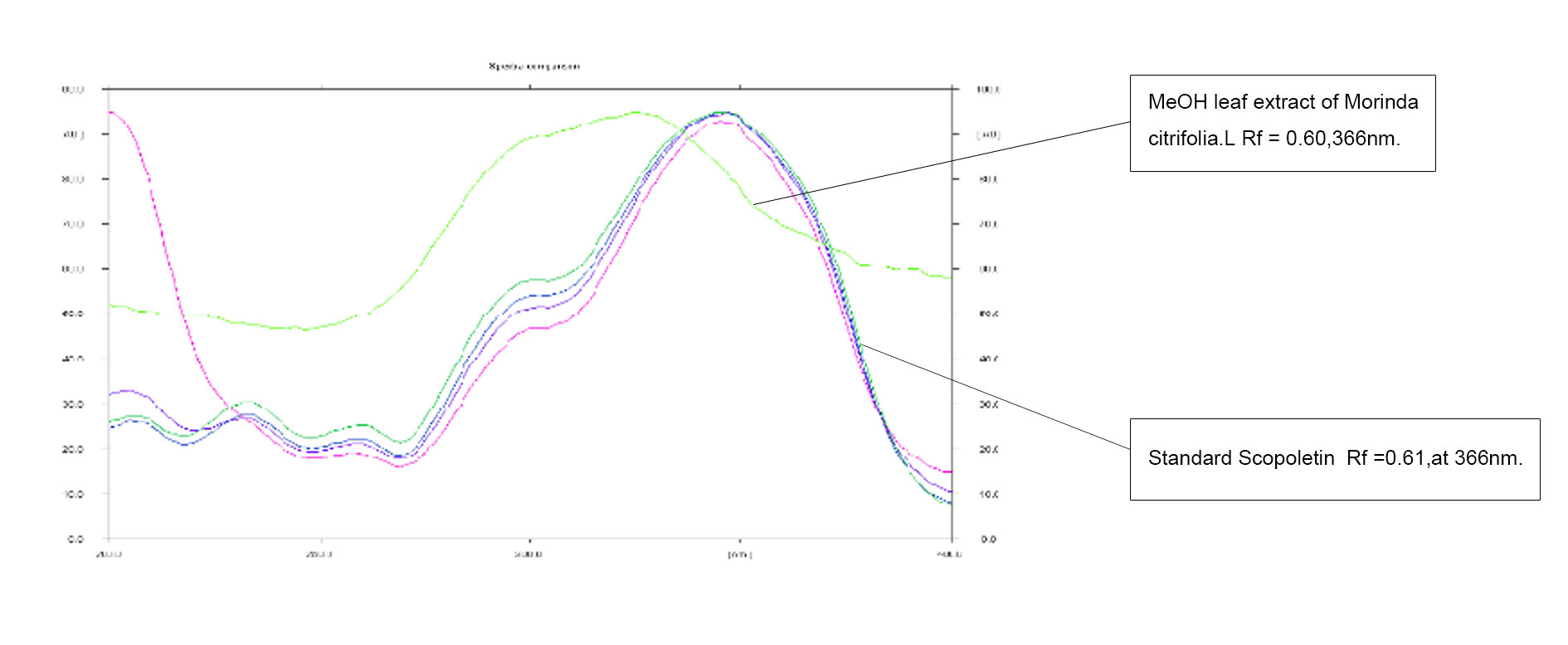 Figure 3. Overlay of the methanol extract of leaf M.citrifolia and standard Scopoletin.
Figure 3. Overlay of the methanol extract of leaf M.citrifolia and standard Scopoletin.
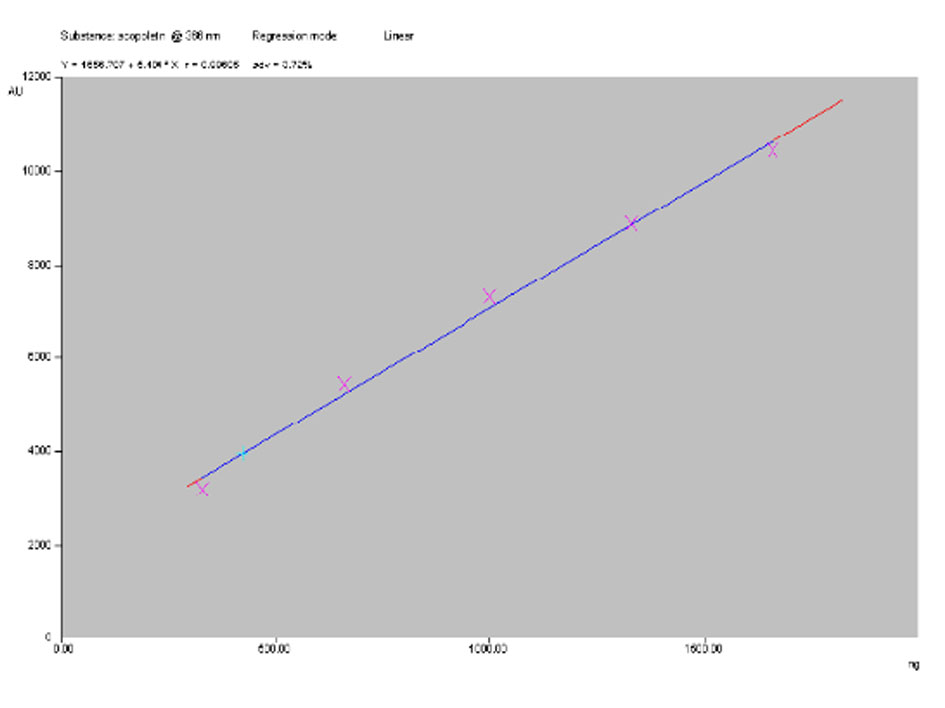 Figure 4. Linearity curve of standard Scopoletin and leaf methanol extract.
Figure 4. Linearity curve of standard Scopoletin and leaf methanol extract.
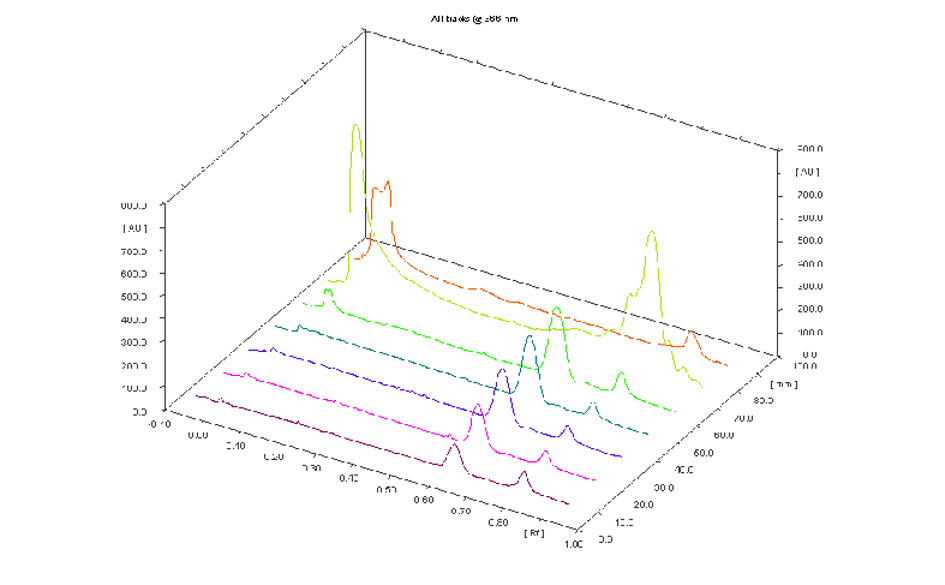 Figure 5. 3D picture standard Scopoletin and leaf methanol extract.
Figure 5. 3D picture standard Scopoletin and leaf methanol extract.
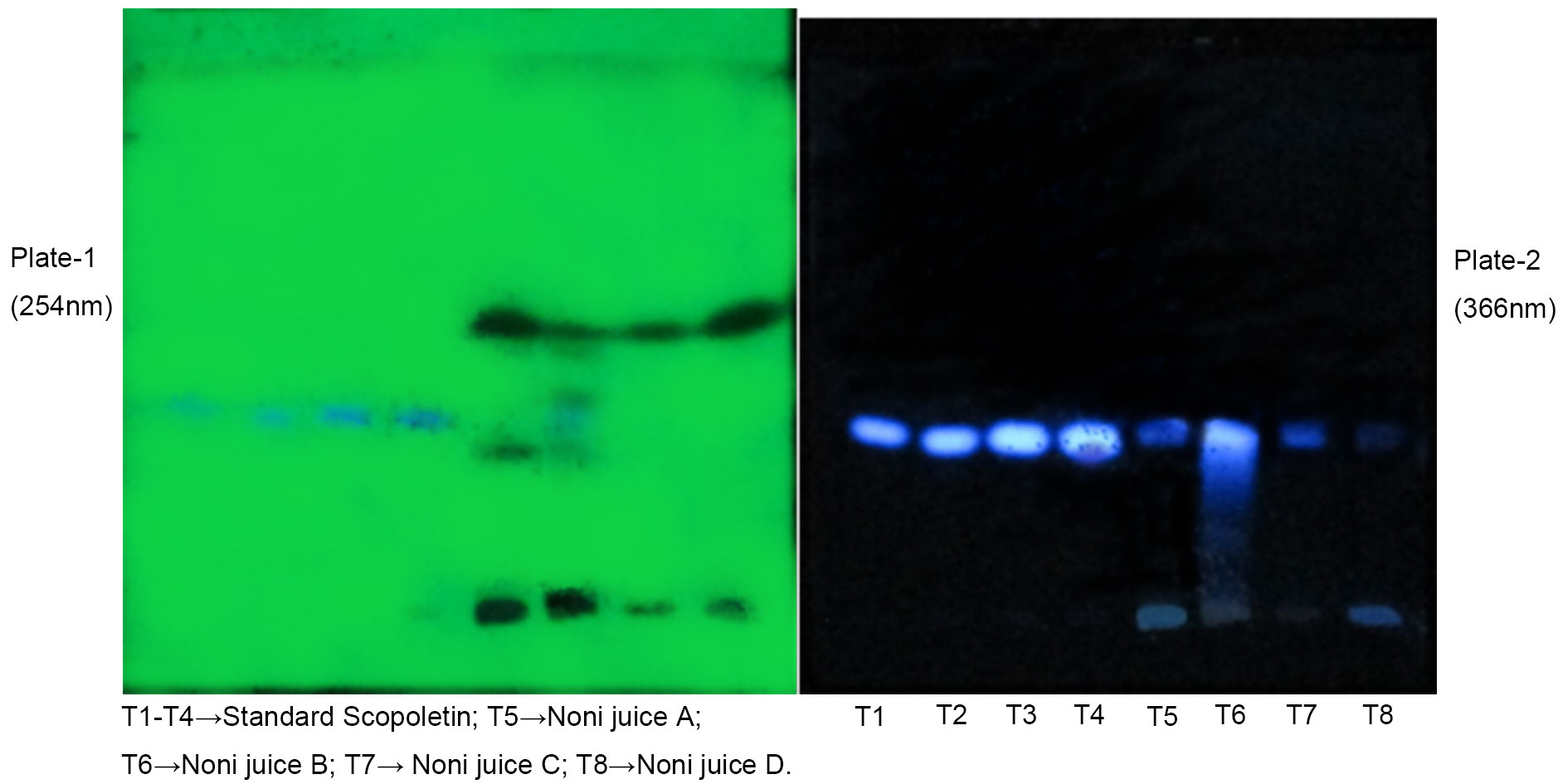 Figure 6. Chromatograms for standard and Noni juices.
Figure 6. Chromatograms for standard and Noni juices.
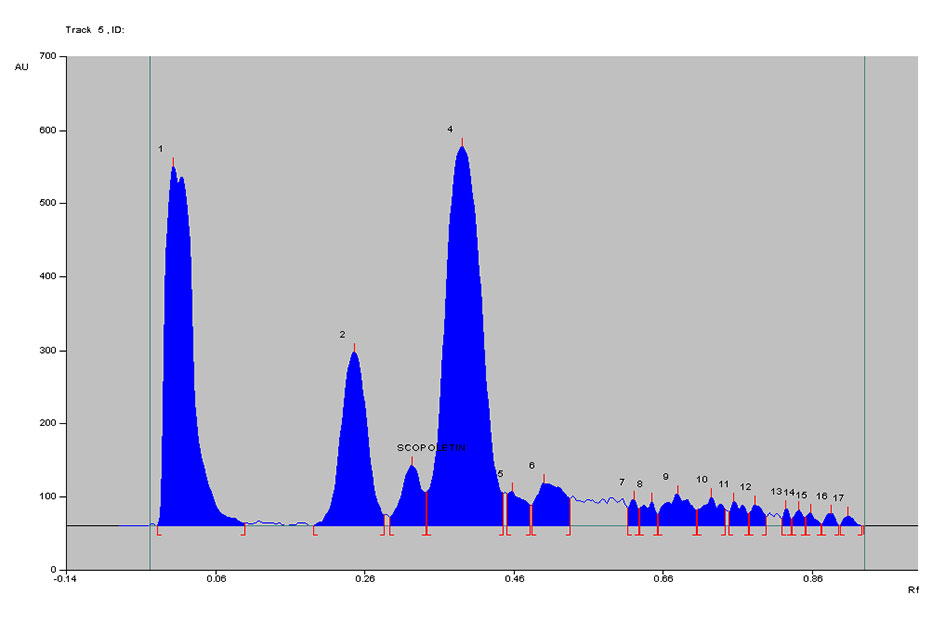 Figure 7. Densitogram of Noni juice A at 366 nm.
Figure 7. Densitogram of Noni juice A at 366 nm.
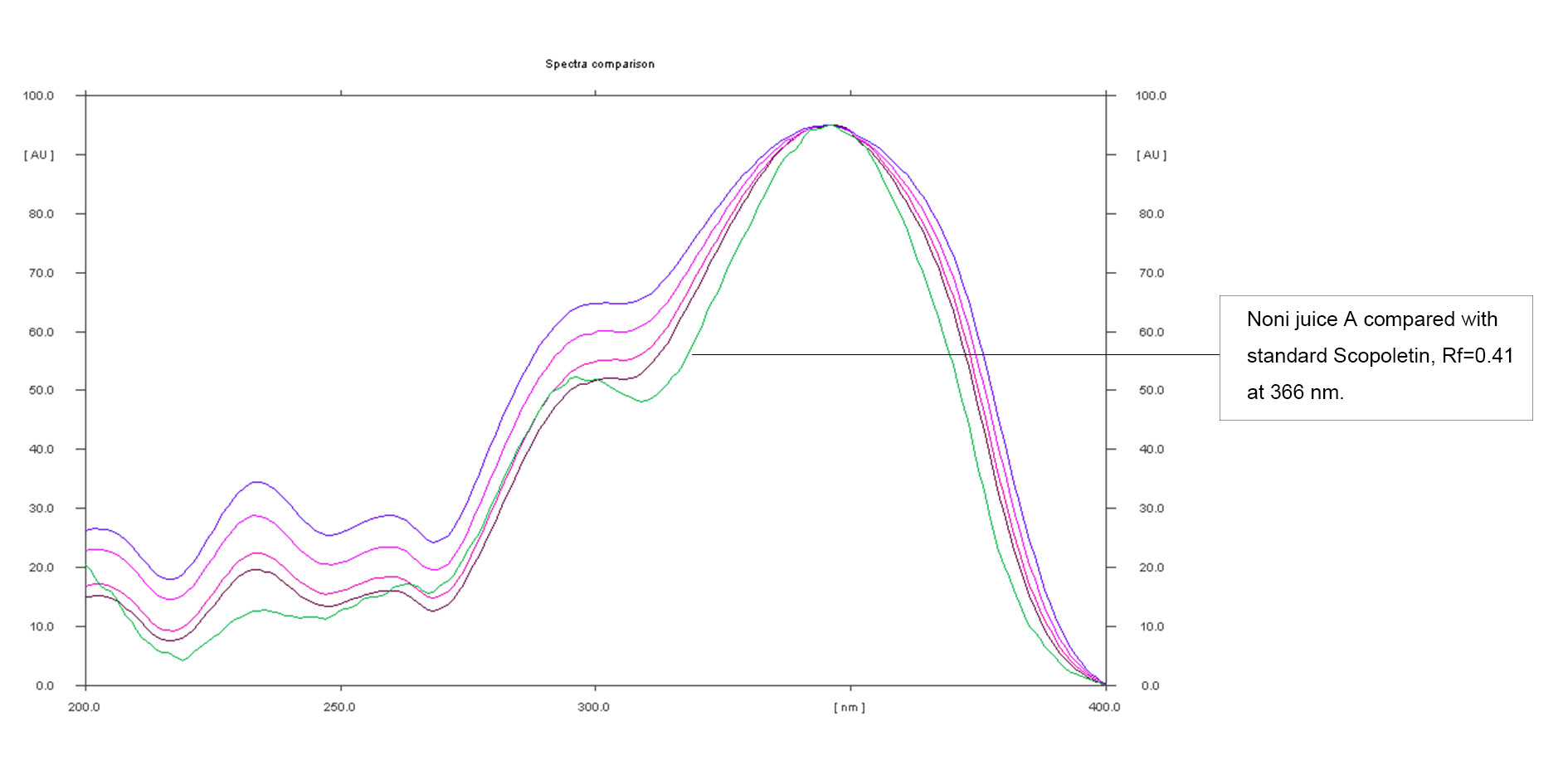 Figure 8. Overlay of Noni juice A and standard Scopoletin.
Figure 8. Overlay of Noni juice A and standard Scopoletin.
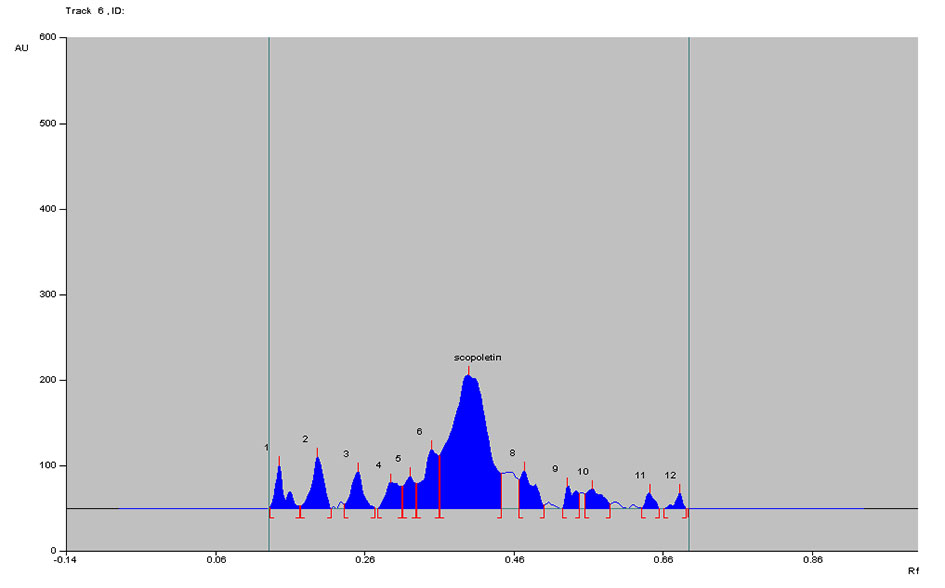 Figure 9. Densitogram of Noni juice B at 366 nm.
Figure 9. Densitogram of Noni juice B at 366 nm.
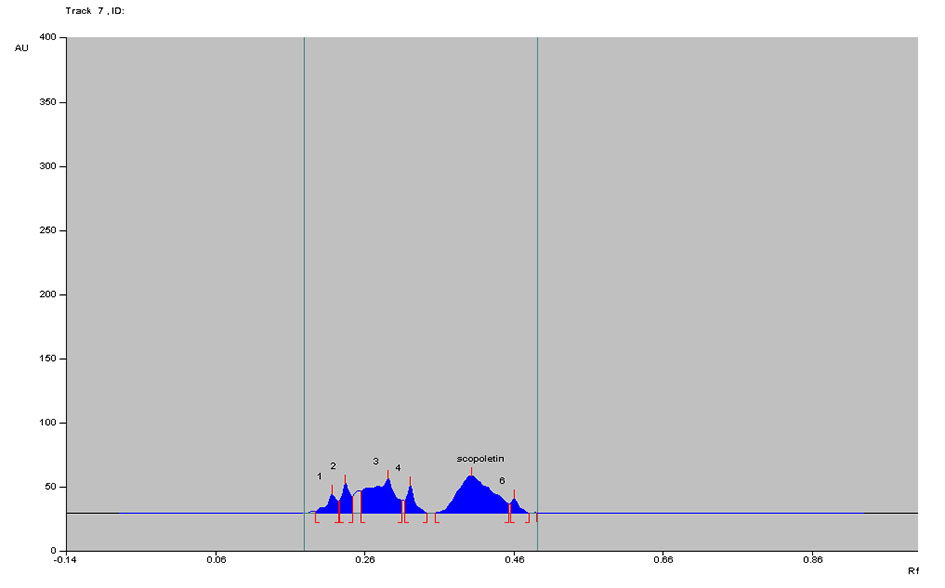 Figure 10. Densitogram of Noni juice C at 366 nm.
Figure 10. Densitogram of Noni juice C at 366 nm.
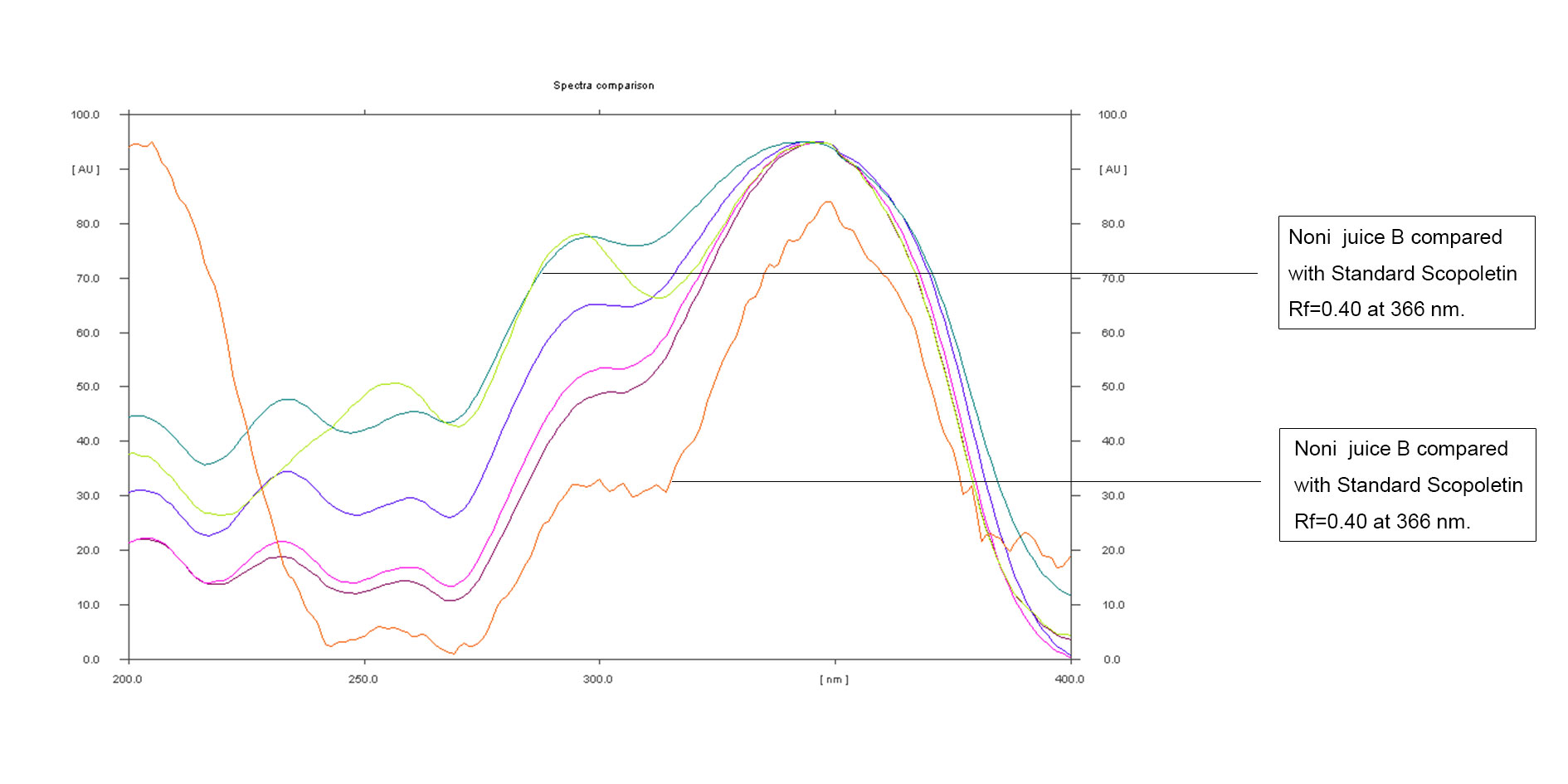 Figure 11. Overlay of Noni juice B and Noni juice C with the standard Scopoletin at 366nm.
Figure 11. Overlay of Noni juice B and Noni juice C with the standard Scopoletin at 366nm.
HPTLC is a simple investigational method for the separation and quantification of natural products. Several attempts were made with various mobile phases in HPTLC to elute Scopoletin, and Toluene: ethyl acetate: Glacial acetic acid (7.5: 2.5: 0.1) was found to be a suitable mobile phase, confirmed by the presence of blue fluorescence in the UV chamber at 254 nm and 366 nm.
However, it's important to note that the quantity of Scopoletin in Noni products can vary significantly. Factors such as temperature, seasonality, and variations in the manufacturing process can all influence the final composition of Noni extracts. Additionally, the inclusion of other botanical ingredients, such as Aloe vera and Garcinia cambogia, in Noni formulations can further complicate the analysis and affect the presence of Scopoletin.
Despite these challenges, the present investigation confirms that both the leaves and fruit juices of Morinda citrifolia contain Scopoletin. This underscores the potential of Noni as a source of this bioactive compound and highlights the importance of standardization in herbal product development.
The quantity of Scopoletin in different brands of Noni juices may vary due to changes in temperature, season, and the manufacturing process. Noni juice sample D did not show the presence of Scopoletin due to the combination of Noni with Aloe vera and Garcinia cambogia. The present investigation revealed that the leaves and fruit juices of Morinda citrifolia contain Scopoletin. The developed HPTLC method would be useful for the isolation and quantification of Scopoletin from Morinda citrifolia and other plants.
In summary, while the presence of Scopoletin in Noni extracts is a promising finding, further comprehensive investigations are necessary to fully understand its therapeutic properties and potential applications. Through a multidisciplinary approach encompassing in vitro, in vivo, mechanistic, and clinical studies, researchers can unlock the full therapeutic potential of this bioactive compound and contribute to the development of effective herbal medicines.
We thank the Principal, Faculty of Pharmacy and KMCH College of Pharmacy, and all the students for their support during this research.
Ethics approval
The ethical committee of the KMCH College of Pharmacy approved the study and written consent was taken for the research work.
Data availability
The Data will be available upon request.
Funding
The authors did not receive any funding or support to report.
Authors’ contribution
We thank Dr. K. Suresh Kumar and Dr. R. Arivukkarasu for critically reviewing the research article thoroughly. and I thank Ms. Sureka for her continuous support.
Competing interests
The authors have reported no conflicts of interest.
- Mohd Zin Z, Abdul Hamid A, Osman A, Saari N: Antioxidative activities of chromatographic fractions obtained from root, fruit, and leaf of Mengkudu (Morinda citrifolia L.) Food Chem 2018, 94: 169-178.
- Assi RA, Darwis Y, Abdulbaqi IM, Khan AA, Vuanghao L, Laghari MH: Morinda citrifolia (Noni): A comprehensive review on its industrial uses, pharmacological activities, and clinical trials. Arab J Chem 2019, 10(5): 691-670.
- Lv L, Chen H, Ho CT, Sang S: Chemical components of the roots of Noni (Morinda citrifolia) and their cytotoxic effects. Fitoterapia 2019, 82(4): 704-708.
- Shami M: Isolation and Identification of Anthraquinones Extracted from Morinda citrifolia L (Rubiaceae). Ann Chromatogr Sep Tech 2019, 1(3): 1012.
- Samoylenko V, Zhao J, Dunbar DC, Khan IA, Rushing JW, Muhammad I: New constituents from Noni (Morinda citrifolia) Fruit Juice. J Agric Food Chem 2006, 54(17): 6398-6402.
- Deng S, Palu 'K, West BJ, Su CX, Zhou BN, Jensen JC: Lipoxygenase Inhibitory Constituents of the Fruits of Noni (Morinda citrifolia) Collected in Tahiti. J Nat Prod 2020, 70(5): 859-862.
- Mani JS, Johnson JB, and Naiker M: The Phytochemistry and Anticarcinogenic Activity of Noni Juice. Eng Proc 2021, 11(1): 16.
- Deng SX, West BJ, Jensen CJ, Basar S, Westendorf J: Development and validation of an RP-HPLC method for the analysis of anthraquinones in noni fruits and leaves. Food Chem 2021, 116: 505-508.
- Desai AG, Qazi GN, Ganju RK, El-Tamer M, Singh J, Saxena AK, Bedi YS, Taneja SC, Bhat HK: Medicinal Plants and Cancer Chemoprevention. Curr Drug Metab 2020, 9(7): 581-591.
- Roy A, Ahuja S, Bharadvaja N: A Review on Medicinal Plants against Cancer. J Plant Sci Agric Res 2019, 2(1): 008.
- Arunachalam SS, Shetty AP, Panniyadi N, Meena C, Kumari J, Rani B, Das P, Kumari S: Study on knowledge of chemotherapy’s adverse effects and their self-care ability to manage - The cancer survivors impact. Clin Epidemiology Glob Health 2021, 11: 100765.
- Sharma K, Pachauri SD, Khandelwal K, Ahmad H, Arya A, Biala P, Agrawal S, Pandey RR, Srivastava A, Srivastav A, Saxena JK, Dwivedi AK: Anticancer Effects of Extracts from the Fruit of Morinda citrifolia (Noni) in Breast Cancer Cell Lines. Drug Res 2019, 66(03): 141-147.
- Thani W, Vallisuta O, Siripong P, Ruangwises N: Anti-proliferative and antioxidative activities of Thai noni/Yor (Morinda citrifolia Linn.) leaf extract. Southeast Asian J Trop Med Public Health 2019, 41(2): 482-489.
- Li J, Chang LC, Wall M, Wong DK, Yu X, Wei Y: Antitumor activity of fermented noni exudates and its fractions. Mol Clin Oncol 2019, 1(1): 161-164.
- Nualsanit T, Rojanapanthu P, Gritsanapan W, Lee SH, Lawson D, Baek SJ: Damnacanthal, a Noni component, exhibits anti-tumorigenic activity in human colorectal cancer cells. J Nutr Biochem 2019, 23(8): 915-923.
- Huang DS, Shen KZ, Wei JF, Liang TB, Zheng SS, Xie HY: Specific COX-2 Inhibitor NS398 Induces Apoptosis in Human Liver Cancer Cell Line Hepg2 Through BCL-2. World J Gastroenterol 2019, 11(2): 204-207.
- Tian Q, Wang L, Sun X, Zeng F, Pan Q, Xue M: Scopoletin exerts anticancer effects on human cervical cancer cell lines by triggering apoptosis, cell cycle arrest, inhibition of cell invasion, and PI3K/AKT signaling pathway. J BUON 2019, 24(3): 997-1002.
- Li CL, Han XC, Zhang H, Wu JS, and Li B: Effect of Scopoletin on Apoptosis and Cell Cycle Arrest in Human Prostate Cancer Cells In vitro Trop. J Pharm Res 2020, 14 (4): 611-617.
- Rajivgandhi G, Saravanan K, Ramachandran G, Li JL, Yin L, Quero F, Alharbi NS, Kadaikunnan S, Khaled JM, Manoharan N, et al: Enhanced anti-cancer activity of chitosan loaded Morinda citrifolia essential oil against A549 human lung cancer cells. Int J Biol Macromol 2020, 164: 4010-4021.
- Anekpankul T, Goto M, Sasaki M, Pavasant P, Shotipruk A: Extraction of anti-cancer damnacanthal from roots of Morinda citrifolia by subcritical water. Sep Purif Technol 2020, 55(3): 343-349.
- Aziz MY, Omar AR, Subramani T, Yeap SK, Ho WY, Ismail NH, Ahmad S, Alitheen NB: Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF-7 breast cancer cells. Oncol Lett 2019, 7(5): 1479-1484.
- Solomon, Neil: the Noni Phenomenon. Direct Source Publishing, Utah. 2nd edition 2019.
- Jayaram S, Govindaraj S, Ganesan A, Muthu R, Appusamy J, Aiyalu R, Ramasamy A: Estimation of Phytoconstituents and Comparative Evaluation of Anti-Obesity Activity of Ayurvedic and Homeopathic Medicines in High Fat Diet Animal Model Indo. American J Pharm Res 2019, 8(3): 1547-1558.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript