Review Article | Open Access
Time matters: chrono-pharmacotherapy as precision medicine
Samir Arabi1, Sajjad Ahmad1
1Department of Biochemistry, Shaheed Salman Building, Islamic Azad University ZIP area 11, Azarshahr Street, Karimkhan-e-Zand Avenue, Tehran 1477893780, Iran.
Correspondence: Sajjad Ahmad (Department of Biochemistry, Shaheed Salman Building, Islamic Azad University ZIP area 11, Azarshahr Street, Karimkhan-e-Zand Avenue, Tehran 1477893780, Iran; E-mail: ahmadsajjad@hotmail.com).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2025, 5: 11-21. https://doi.org/10.32948/ajpt.2025.01.20
Received: 02 Jan 2025 | Accepted: 10 Feb 2025 | Published online: 20 Feb 2025
Key words circadian rhythms, molecular clock, pharmacodynamics, chrono-pharmacotherapy, precision medicine
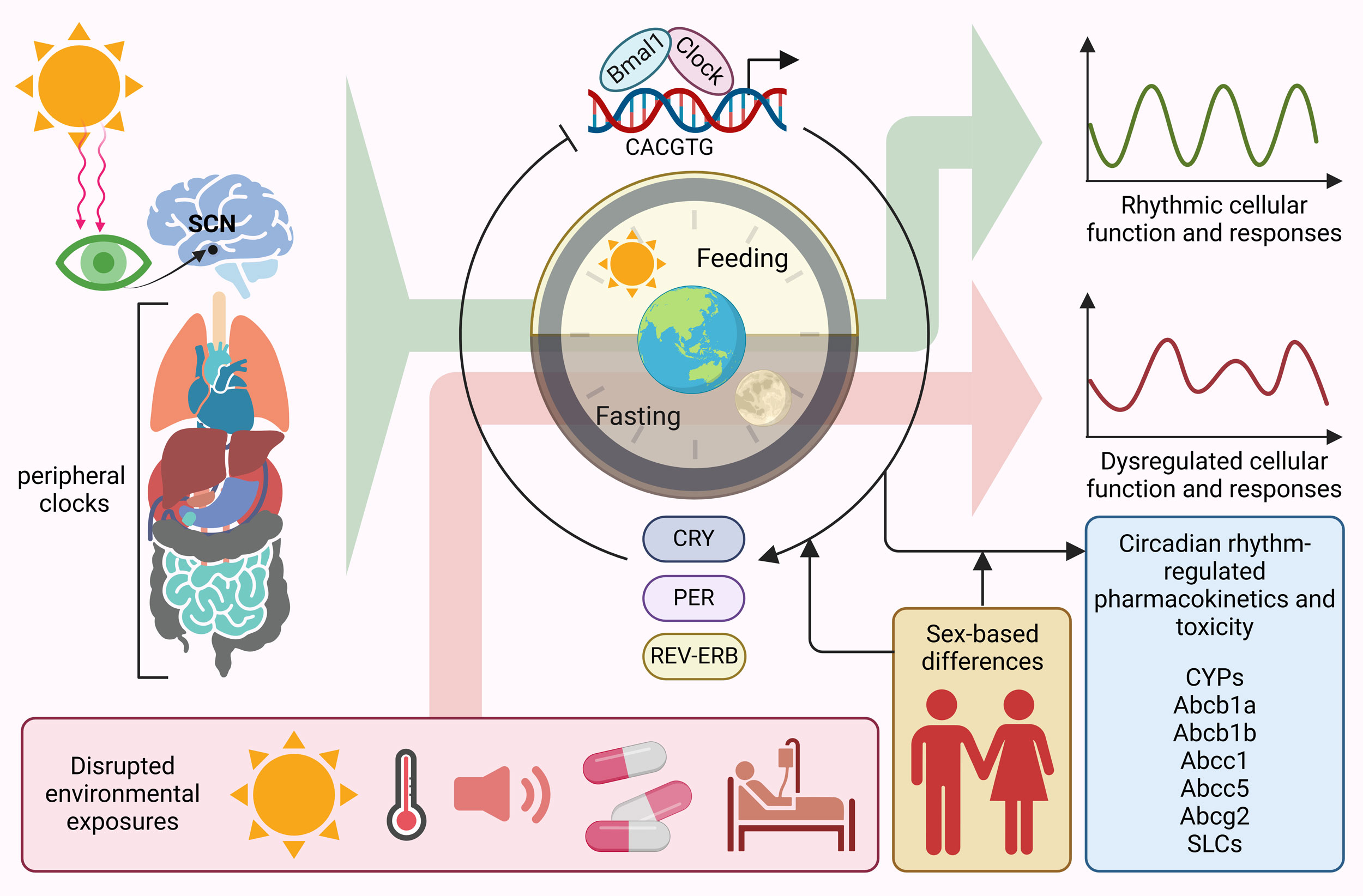 Figure 1. Circadian rhythm-regulated pharmacokinetics and toxicity. Circadian clock in primarily regulated by suprachiasmatic nuclei (SCN), synchronizing the peripheral clocks throughout the body. Bmal1 and Clock are the master regulator of circadian rhythm throughout the body leading rhythmic cellular function and responses. Disrupted environmental exposures such as sunlight, heat, sound, diseases and treatment plans dysregulate circadian rhythms. In addition, sex-based differences also contribute to alterations in circadian rhythms. Different pharmacokinetic and toxicity associated genes are tightly regulated by circadian clock, expression rhythmic expressions.
Figure 1. Circadian rhythm-regulated pharmacokinetics and toxicity. Circadian clock in primarily regulated by suprachiasmatic nuclei (SCN), synchronizing the peripheral clocks throughout the body. Bmal1 and Clock are the master regulator of circadian rhythm throughout the body leading rhythmic cellular function and responses. Disrupted environmental exposures such as sunlight, heat, sound, diseases and treatment plans dysregulate circadian rhythms. In addition, sex-based differences also contribute to alterations in circadian rhythms. Different pharmacokinetic and toxicity associated genes are tightly regulated by circadian clock, expression rhythmic expressions.
To overcome resistance mechanisms, anticancer drugs are commonly administered in combination regimens. Both experimental studies and clinical data suggest that each drug should be delivered at best effective circadian phase [49]. Current research efforts focus on translational strategies aimed at identifying factors that can stratify patients for chronotherapy, including sex-based differences and circadian biomarkers, while also incorporating systems medicine principles. For instance, synchronized Caco-2 cell culture models have been employed to investigate the molecular and cellular chronopharmacology of irinotecan, a key drug in gastrointestinal cancer treatment [27]. Studies have demonstrated that silencing Bmal1 abolished the circadian fluctuations in irinotecan’s bioactivation to SN38, its interaction with TOP1-DNA, and the subsequent induction of apoptosis. Similarly, when Bmal1 and its molecular partner Clock were either suppressed, knocked down, or mutated, cyclophosphamide toxicity remained fixed at its most harmful circadian phase in male mice [50]. These findings emphasize the potential for artificial intelligence-driven models to integrate experimental and clinical data, paving the way for personalized chronotherapy [51]. Despite mounting evidence that chemotherapy tolerability differs between sexes, no formal guidelines exist for sex-specific drug dosing or scheduling [52]. Female patients tend to experience greater adverse effects when receiving time-unspecified doses of 5-FU and irinotecan compared to male patients [53, 54]. Research suggests that sex-related differences in cancer chronotherapy are becoming increasingly apparent, largely due to clinical observations [55]. For instance, studies have revealed that when 5-FU is administered at a constant rate, its plasma concentration exhibits circadian variations, with women showing lower circadian amplitude in 5-FU clearance than men [56]. Moreover, a study involving 210 patients demonstrated that, in cases of diffuse large B-cell lymphoma, female patients, but not male patients, had significantly improved survival rates when rituximab, cyclophosphamide, doxorubicin and vincristine were administered in the afternoon rather than in the morning [57]. Sex-based differences have also been confirmed in preclinical models, particularly regarding irinotecan-induced systemic and organ toxicities, as well as its plasma pharmacokinetics in mice. Statistical modeling has identified the reciprocal transcriptional dynamics of Rev-Erbα and Bmal1 as key regulators of sex-specific variations in response to irinotecan treatment [58]. Although research in this area is still in its early stages, these findings are consistent with clinical observations, highlighting the need for further prospective studies to explore how sex and circadian timing interact to refine cancer chronotherapy.
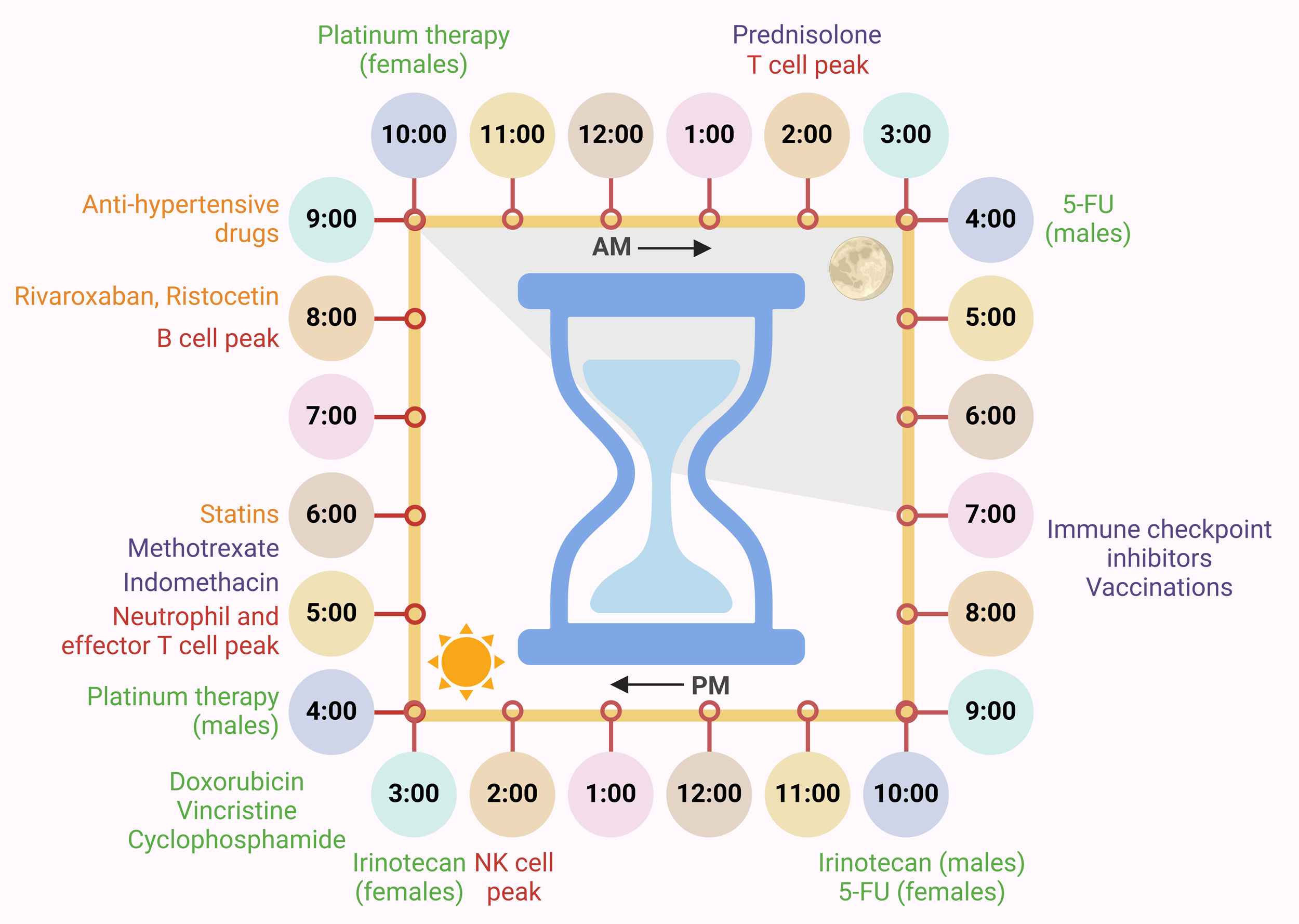 Figure 2. Circadian clock-driven immune system regulation and responses towards treatments. Circadian clock-driven immune system regulations entailing peak timings of various immune responses in the body (red) are listed along with optimized timing for various chemo- (green), cardiovascular (orange) and immunotherapies and anti-inflammatory drugs (purple).
Figure 2. Circadian clock-driven immune system regulation and responses towards treatments. Circadian clock-driven immune system regulations entailing peak timings of various immune responses in the body (red) are listed along with optimized timing for various chemo- (green), cardiovascular (orange) and immunotherapies and anti-inflammatory drugs (purple).
Chronopharmacological research has also reinforced the beneficial impact of medication timing for the treatment of both corticosteroids and nonsteroidal anti-inflammatory drugs. For instance, in individuals with osteoarthritis, taking the sustained-release version of indomethacin in the evening rather than in the morning led to nearly four times lower toxicity and twice the therapeutic efficacy [96]. Likewise, in patients with rheumatoid arthritis, administering short-acting prednisolone around 2:00 AM was found to be most effective in reducing morning stiffness [97]. To improve adherence to treatment without requiring patients to wake up for nighttime dosing, researchers developed a modified-release formulation of prednisone. When taken at 10:00 PM, this formulation delayed drug release by approximately four hours, synchronizing with peak levels of circulating cytokines responsible for joint stiffness [98, 99]. Similarly, methotrexate, an anti-inflammatory dihydrofolate reductase inhibitor, showed superior effectiveness in rheumatoid arthritis patients when taken in the evening compared to the conventional thrice-daily regimen [100]. Moreover, the relevance of circadian-based drug administration is not limited to inflammatory diseases. Studies indicate that aligning the timing of central nervous system–acting medications with circadian rhythms can improve therapeutic outcomes in both psychiatric and neurological disorders [101, 102].
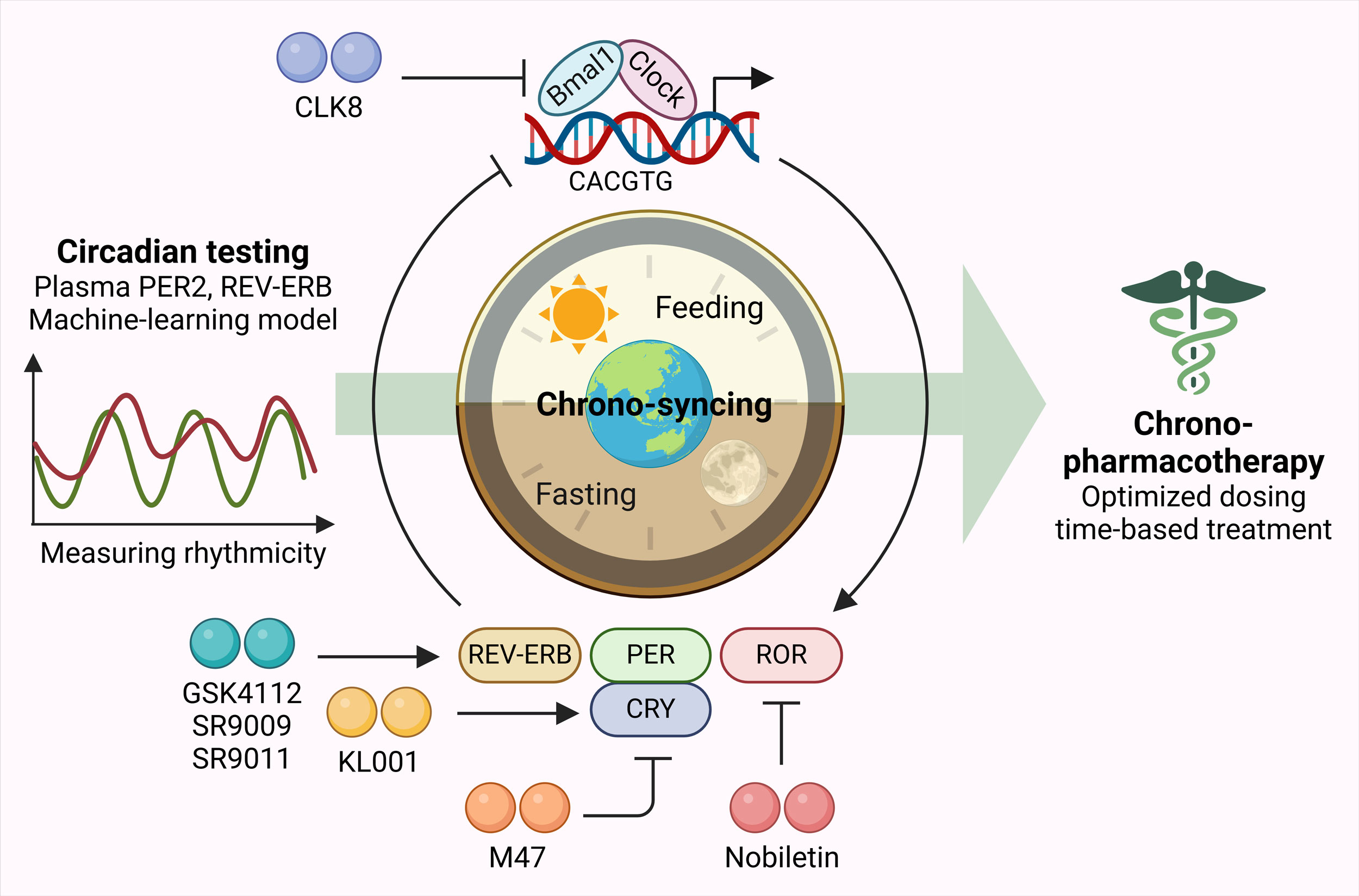 Figure 3. Chrono-pharmacotherapy as precision medicine. Plasma PER2, REV-ERB levels along with machine-learning models can serve as circadian testing tools to measure rhythmicity. Based on which, chrono-syncing can be performed using different therapeutic agents targeting genes involved in regulating circadian clock. This can help in devising optimized dosing time-based treatment plans as precision medicine.
Figure 3. Chrono-pharmacotherapy as precision medicine. Plasma PER2, REV-ERB levels along with machine-learning models can serve as circadian testing tools to measure rhythmicity. Based on which, chrono-syncing can be performed using different therapeutic agents targeting genes involved in regulating circadian clock. This can help in devising optimized dosing time-based treatment plans as precision medicine.
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
Samir Arabi contributed to draft, critical revision of the article, table making, and figure drawing; Sajjad Ahmad checked and revised the manuscript and approved the final submission.
Competing interests
The authors declare no competing interests.
- Fagiani F, Di Marino D, Romagnoli A, Travelli C, Voltan D, Di Cesare Mannelli L, Racchi M, Govoni S, Lanni C: Molecular regulations of circadian rhythm and implications for physiology and diseases. Sig Transduct Target Ther 2022, 7(1): 41.
- Hastings MH, Reddy AB, Maywood ES: A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci 2003, 4(8): 649-661.
- Lévi F, Okyar A, Dulong S, Innominato PF, Clairambault J: Circadian timing in cancer treatments. Annu Rev Pharmacol Toxicol 2010, 50: 377-421.
- Dallmann R, Okyar A, Lévi F: Dosing-Time Makes the Poison: Circadian Regulation and Pharmacotherapy. Trends Mol Med 2016, 22(5): 430-445.
- Lévi FA, Okyar A, Hadadi E, Innominato PF, Ballesta A: Circadian Regulation of Drug Responses: Toward Sex-Specific and Personalized Chronotherapy. Annu Rev Pharmacol Toxicol 2024, 64: 89-114.
- Ayyar VS, Sukumaran S: Circadian rhythms: influence on physiology, pharmacology, and therapeutic interventions. J Pharmacokinet Pharmacodyn 2021, 48(3): 321-338.
- Amorós-Figueras G, Jorge E, Alonso-Martin C, Traver D, Ballesta M, Bragós R, Rosell-Ferrer J, Cinca J: Endocardial infarct scar recognition by myocardial electrical impedance is not influenced by changes in cardiac activation sequence. Heart Rhythm 2018, 15(4): 589-596.
- Hesse J, Malhan D, Yalҫin M, Aboumanify O, Basti A, Relógio A: An Optimal Time for Treatment-Predicting Circadian Time by Machine Learning and Mathematical Modelling. Cancers (Basel) 2020, 12(11): 3103.
- Talamanca L, Naef F: How to tell time: advances in decoding circadian phase from omics snapshots. F1000Res 2020, 9: F1000 Faculty Rev-1150.
- Dose B, Yalçin M, Dries SPM, Relógio A: TimeTeller for timing health: The potential of circadian medicine to improve performance, prevent disease and optimize treatment. Front Digit Health 2023, 5: 1157654.
- Cederroth CR, Albrecht U, Bass J, Brown SA, Dyhrfjeld-Johnsen J, Gachon F, Green CB, Hastings MH, Helfrich-Förster C, Hogenesch JB et al: Medicine in the Fourth Dimension. Cell Metab 2019, 30(2): 238-250.
- Schwartzberg L, Kim ES, Liu D, Schrag D: Precision Oncology: Who, How, What, When, and When Not? Am Soc Clin Oncol Educ Book 2017, 37: 160-169.
- Levi F, Schibler U: Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol 2007, 47: 593-628.
- Hesse J, Martinelli J, Aboumanify O, Ballesta A, Relógio A: A mathematical model of the circadian clock and drug pharmacology to optimize irinotecan administration timing in colorectal cancer. Comput Struct Biotechnol J 2021, 19: 5170-5183.
- Filipski E, King VM, Etienne MC, Li X, Claustrat B, Granda TG, Milano G, Hastings MH, Lévi F: Persistent twenty-four hour changes in liver and bone marrow despite suprachiasmatic nuclei ablation in mice. Am J Physiol Regul Integr Comp Physiol 2004, 287(4): R844-851.
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ et al: PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A 2004, 101(15): 5339-5346.
- Finger AM, Kramer A: Mammalian circadian systems: Organization and modern life challenges. Acta Physiol (Oxf) 2021, 231(3): e13548.
- Daiber A, Frenis K, Kuntic M, Li H, Wolf E, Kilgallen AB, Lecour S, Van Laake LW, Schulz R, Hahad O et al: Redox Regulatory Changes of Circadian Rhythm by the Environmental Risk Factors Traffic Noise and Air Pollution. Antioxid Redox Signal 2022, 37(10-12): 679-703.
- Fougeray T, Polizzi A, Régnier M, Fougerat A, Ellero-Simatos S, Lippi Y, Smati S, Lasserre F, Tramunt B, Huillet M et al: The hepatocyte insulin receptor is required to program the liver clock and rhythmic gene expression. Cell Rep 2022, 39(2): 110674.
- Takahashi JS: Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet 2017, 18(3): 164-179.
- Robles MS, Humphrey SJ, Mann M: Phosphorylation Is a Central Mechanism for Circadian Control of Metabolism and Physiology. Cell Metab 2017, 25(1): 118-127.
- Anderson ST, FitzGerald GA: Sexual dimorphism in body clocks. Science 2020, 369(6508): 1164-1165.
- Talamanca L, Gobet C: Sex-dimorphic and age-dependent organization of 24-hour gene expression rhythms in humans. Science 2023, 379(6631): 478-483.
- Wucher V, Sodaei R, Amador R, Irimia M: Day-night and seasonal variation of human gene expression across tissues. PLoS Biol 2023, 21(2): e3001986.
- Dobrek L: Chronopharmacology in Therapeutic Drug Monitoring-Dependencies between the Rhythmics of Pharmacokinetic Processes and Drug Concentration in Blood. Pharmaceutics 2021, 13(11): 1915.
- Dong D, Yang D, Lin L, Wang S, Wu B: Circadian rhythm in pharmacokinetics and its relevance to chronotherapy. Biochem Pharmacol 2020, 178: 114045.
- Dulong S, Ballesta A, Okyar A, Lévi F: Identification of Circadian Determinants of Cancer Chronotherapy through In Vitro Chronopharmacology and Mathematical Modeling. Mol Cancer Ther 2015, 14(9): 2154-2164.
- Wada E, Koyanagi S, Kusunose N, Akamine T, Masui H, Hashimoto H, Matsunaga N, Ohdo S: Modulation of peroxisome proliferator-activated receptor-α activity by bile acids causes circadian changes in the intestinal expression of Octn1/Slc22a4 in mice. Mol Pharmacol 2015, 87(2): 314-322.
- Pácha J, Balounová K, Soták M: Circadian regulation of transporter expression and implications for drug disposition. Expert Opin Drug Metab Toxicol 2021, 17(4): 425-439.
- Zhang YK, Yeager RL, Klaassen CD: Circadian expression profiles of drug-processing genes and transcription factors in mouse liver. Drug Metab Dispos 2009, 37(1): 106-115.
- Radzialowski FM, Bousquet WF: Daily rhythmic variation in hepatic drug metabolism in the rat and mouse. J Pharmacol Exp Ther 1968, 163(1): 229-238.
- Nair V, Casper R: The influence of light on daily rhythm in hepatic drug metabolizing enzymes in rat. Life Sci 1969, 8(23): 1291-1298.
- Gachon F, Olela FF, Schaad O, Descombes P, Schibler U: The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab 2006, 4(1): 25-36.
- Nicolaides NC, Chrousos GP: Sex differences in circadian endocrine rhythms: Clinical implications. Eur J Neurosci 2020, 52(1): 2575-2585.
- Soldin OP, Chung SH, Mattison DR: Sex differences in drug disposition. J Biomed Biotechnol 2011, 2011: 187103.
- Okyar A, Piccolo E, Ahowesso C, Filipski E, Hossard V, Guettier C, La Sorda R, Tinari N, Iacobelli S, Lévi F: Strain- and sex-dependent circadian changes in abcc2 transporter expression: implications for irinotecan chronotolerance in mouse ileum. PLoS One 2011, 6(6): e20393.
- Okyar A, Kumar SA, Filipski E, Piccolo E, Ozturk N, Xandri-Monje H, Pala Z, Abraham K, Gomes A, Orman MN et al: Sex-, feeding-, and circadian time-dependency of P-glycoprotein expression and activity - implications for mechanistic pharmacokinetics modeling. Sci Rep 2019, 9(1): 10505.
- Cui YJ, Cheng X, Weaver YM, Klaassen CD: Tissue distribution, gender-divergent expression, ontogeny, and chemical induction of multidrug resistance transporter genes (Mdr1a, Mdr1b, Mdr2) in mice. Drug Metab Dispos 2009, 37(1): 203-210.
- Ando H, Yanagihara H, Sugimoto K, Hayashi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A: Daily rhythms of P-glycoprotein expression in mice. Chronobiol Int 2005, 22(4): 655-665.
- Du K, Williams CD, McGill MR, Jaeschke H: Lower susceptibility of female mice to acetaminophen hepatotoxicity: Role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol Appl Pharmacol 2014, 281(1): 58-66.
- Dulong S, de Souza LEB: Sex and Circadian Timing Modulate Oxaliplatin Hematological and Hematopoietic Toxicities. Pharmaceutics 2022, 14(11): 2465.
- Masubuchi Y, Nakayama J, Watanabe Y: Sex difference in susceptibility to acetaminophen hepatotoxicity is reversed by buthionine sulfoximine. Toxicology 2011, 287(1-3): 54-60.
- Marcu LG: Developments on tumour site-specific chrono-oncology towards personalised treatment. Crit Rev Oncol Hematol 2022, 179: 103803.
- Printezi MI, Kilgallen AB, Bond MJG, Štibler U, Putker M, Teske AJ, Cramer MJ, Punt CJA, Sluijter JPG, Huitema ADR et al: Toxicity and efficacy of chronomodulated chemotherapy: a systematic review. Lancet Oncol 2022, 23(3): e129-e143.
- Siegel RL, Wagle NS: Colorectal cancer statistics, 2023. CA Cancer J Clin 2023, 73(3): 233-254.
- Zhang PX, Jin F, Li ZL, Wu WL, Li YY, Long JH, Chen GY, Chen XX, Gan JY, Gong XY et al: A randomized phase II trial of induction chemotherapy followed by cisplatin chronotherapy versus constant rate delivery combined with radiotherapy. Chronobiol Int 2018, 35(2): 240-248.
- Li J, Chen R, Ji M, Zou SL, Zhu LN: Cisplatin-based chronotherapy for advanced non-small cell lung cancer patients: a randomized controlled study and its pharmacokinetics analysis. Cancer Chemother Pharmacol 2015, 76(3): 651-655.
- Kumar SA, Needham RJ, Abraham K, Bridgewater HE, Garbutt LA, Xandri-Monje H, Dallmann R, Perrier S, Sadler PJ, Lévi F: Dose- and time-dependent tolerability and efficacy of organo-osmium complex FY26 and its tissue pharmacokinetics in hepatocarcinoma-bearing mice. Metallomics 2021, 13(2): mfaa003.
- Innominato PF, Lévi FA, Bjarnason GA: Chronotherapy and the molecular clock: Clinical implications in oncology. Adv Drug Deliv Rev 2010, 62(9-10): 979-1001.
- Gorbacheva VY, Kondratov RV, Zhang R, Cherukuri S, Gudkov AV, Takahashi JS, Antoch MP: Circadian sensitivity to the chemotherapeutic agent cyclophosphamide depends on the functional status of the CLOCK/BMAL1 transactivation complex. Proc Natl Acad Sci U S A 2005, 102(9): 3407-3412.
- Ballesta A, Innominato PF, Dallmann R, Rand DA, Lévi FA: Systems Chronotherapeutics. Pharmacol Rev 2017, 69(2): 161-199.
- Wagner AD, Oertelt-Prigione S, Adjei A, Buclin T, Cristina V, Csajka C, Coukos G, Dafni U, Dotto GP, Ducreux M et al: Gender medicine and oncology: report and consensus of an ESMO workshop. Ann Oncol 2019, 30(12): 1914-1924.
- Cristina V, Mahachie J, Mauer M, Buclin T, Van Cutsem E, Roth A, Wagner AD: Association of Patient Sex With Chemotherapy-Related Toxic Effects: A Retrospective Analysis of the PETACC-3 Trial Conducted by the EORTC Gastrointestinal Group. JAMA Oncol 2018, 4(7): 1003-1006.
- Chansky K, Benedetti J, Macdonald JS: Differences in toxicity between men and women treated with 5-fluorouracil therapy for colorectal carcinoma. Cancer 2005, 103(6): 1165-1171.
- Spitschan M, Santhi N: Sex differences and sex bias in human circadian and sleep physiology research. Elife 2022, 11: e65419.
- Bressolle F, Joulia JM, Pinguet F, Ychou M, Astre C, Duffour J, Gomeni R: Circadian rhythm of 5-fluorouracil population pharmacokinetics in patients with metastatic colorectal cancer. Cancer Chemother Pharmacol 1999, 44(4): 295-302.
- Kim DW, Byun JM, Lee JO, Kim JK, Koh Y: Chemotherapy delivery time affects treatment outcomes of female patients with diffuse large B cell lymphoma. JCI Insight 2023, 8(2): e164767.
- Ahowesso C, Li XM, Zampera S, Peteri-Brunbäck B, Dulong S, Beau J, Hossard V, Filipski E, Delaunay F, Claustrat B et al: Sex and dosing-time dependencies in irinotecan-induced circadian disruption. Chronobiol Int 2011, 28(5): 458-470.
- Chaudhary R, Sharma T, Tantry US, Asgar JA, Kundan P, Duhan S, Gill H, Singh A, Alasadi Y, Gurbel PA: Serial assessment of thrombogenicity and hemodynamics in patients with type II diabetes in a clinical research unit: Evidence for circadian variations in clot formation. J Thromb Thrombolysis 2022, 54(3): 393-400.
- Schved JF, Gris JC, Eledjam JJ: Circadian changes in anticoagulant effect of heparin infused at a constant rate. Br Med J (Clin Res Ed) 1985, 290(6477): 1286.
- Brunner-Ziegler S, Jilma B, Schörgenhofer C, Winkler F, Jilma-Stohlawetz P, Koppensteiner R, Quehenberger P, Seger C, Weigel G, Griesmacher A et al: Comparison between the impact of morning and evening doses of rivaroxaban on the circadian endogenous coagulation rhythm in healthy subjects. J Thromb Haemost 2016, 14(2): 316-323.
- Schoergenhofer C, Schwameis M, Brunner M, Zeitlinger M, Winkler F, Jilma B, Brunner-Ziegler S: Assessing the influence of diurnal variations and selective Xa inhibition on whole blood aggregometry. Scand J Clin Lab Invest 2015, 75(6): 531-536.
- Soulban G, Labrecque G: Circadian rhythms of blood clotting time and coagulation factors II, VII, IX and X in rats. Life Sci 1989, 45(25): 2485-2489.
- Gumz ML, Shimbo D: Toward Precision Medicine: Circadian Rhythm of Blood Pressure and Chronotherapy for Hypertension - 2021 NHLBI Workshop Report. Hypertension 2023, 80(3): 503-522.
- Hermida-Ayala RG, Mojón A, Fernández JR, Smolensky MH, Hermida RC: Ingestion-time differences in the pharmacodynamics of dual-combination hypertension therapies: Systematic review and meta-analysis of published human trials. Chronobiol Int 2022, 39(4): 493-512.
- Ruben MD, Smith DF, FitzGerald GA, Hogenesch JB: Dosing time matters. Science 2019, 365(6453): 547-549.
- Hermida RC, Smolensky MH, Balan H, Castriotta RJ, Crespo JJ, Dagan Y, El-Toukhy S, Fernández JR, FitzGerald GA, Fujimura A et al: Guidelines for the design and conduct of human clinical trials on ingestion-time differences - chronopharmacology and chronotherapy - of hypertension medications. Chronobiol Int 2021, 38(1): 1-26.
- Hermida RC, Mojón A, Fernández JR, Hermida-Ayala RG, Crespo JJ, Ríos MT, Domínguez-Sardiña M, Otero A, Smolensky MH: Elevated asleep blood pressure and non-dipper 24h patterning best predict risk for heart failure that can be averted by bedtime hypertension chronotherapy: A review of the published literature. Chronobiol Int 2023, 40(1): 63-82.
- Irwin MR: Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol 2019, 19(11): 702-715.
- Valenzuela PL, Carrera-Bastos P: Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol 2021, 18(4): 251-275.
- Chou R, Dana T, Blazina I, Daeges M, Jeanne TL: Statins for Prevention of Cardiovascular Disease in Adults: Evidence Report and Systematic Review for the US Preventive Services Task Force. Jama 2016, 316(19): 2008-2024.
- Hamprecht B, Nüssler C, Lynen F: Rhythmic changes of hydroxymethylglutaryl coenzyme a reductase activity in livers of fed and fasted rats. FEBS Lett 1969, 4(2): 117-121.
- Ness GC, Chambers CM: Feedback and hormonal regulation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase: the concept of cholesterol buffering capacity. Proc Soc Exp Biol Med 2000, 224(1): 8-19.
- Izquierdo-Palomares JM, Fernandez-Tabera JM, Plana MN, Añino Alba A, Gómez Álvarez P, Fernandez-Esteban I, Saiz LC, Martin-Carrillo P, Pinar López Ó: Chronotherapy versus conventional statins therapy for the treatment of hyperlipidaemia. Cochrane Database Syst Rev 2016, 11(11): Cd009462.
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B: A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A 2009, 106(50): 21407-21412.
- Lange T, Luebber F: The contribution of sleep to the neuroendocrine regulation of rhythms in human leukocyte traffic. Semin Immunopathol 2022, 44(2) :239-254.
- Ackermann K, Revell VL, Lao O, Rombouts EJ, Skene DJ, Kayser M: Diurnal rhythms in blood cell populations and the effect of acute sleep deprivation in healthy young men. Sleep 2012, 35(7): 933-940.
- Downton P, Early JO: Circadian rhythms in adaptive immunity. Immunology 2020, 161(4): 268-277.
- Lasrado N, Jia T, Massilamany C, Franco R, Illes Z, Reddy J: Mechanisms of sex hormones in autoimmunity: focus on EAE. Biol Sex Differ 2020, 11(1): 50.
- Xie G, Wang X, Zhao A, Yan J, Chen W, Jiang R, Ji J, Huang F, Zhang Y, Lei S et al: Sex-dependent effects on gut microbiota regulate hepatic carcinogenic outcomes. Sci Rep 2017, 7: 45232.
- Elderman M, de Vos P, Faas M: Role of Microbiota in Sexually Dimorphic Immunity. Front Immunol 2018, 9: 1018.
- Zheng D, Liwinski T, Elinav E: Interaction between microbiota and immunity in health and disease. Cell Res 2020, 30(6): 492-506.
- Thaiss CA, Zmora N, Levy M, Elinav E: The microbiome and innate immunity. Nature 2016, 535(7610): 65-74.
- Ursini F, De Giorgi A, D'Onghia M, De Giorgio R: Chronobiology and Chronotherapy in Inflammatory Joint Diseases. Pharmaceutics 2021, 13(11): 1832.
- Guha P, Heatherton KR, O'Connell KP, Alexander IS: Assessing the Future of Solid Tumor Immunotherapy. Biomedicines 2022, 10(3): 655.
- Qian DC, Kleber T, Brammer B, Xu KM, Switchenko JM, Janopaul-Naylor JR, Zhong J, Yushak ML, Harvey RD, Paulos CM et al: Effect of immunotherapy time-of-day infusion on overall survival among patients with advanced melanoma in the USA (MEMOIR): a propensity score-matched analysis of a single-centre, longitudinal study. Lancet Oncol 2021, 22(12): 1777-1786.
- Patel JS, Woo Y, Draper A, Jansen CS: Impact of immunotherapy time-of-day infusion on survival and immunologic correlates in patients with metastatic renal cell carcinoma: a multicenter cohort analysis. J Immunother Cancer 2024, 12(3): e008011.
- Rousseau A, Tagliamento M, Auclin E, Aldea M, Frelaut M, Levy A, Benitez JC, Naltet C, Lavaud P, Botticella A et al: Clinical outcomes by infusion timing of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Eur J Cancer 2023, 182: 107-114.
- Centanni M, Moes D, Trocóniz IF, Ciccolini J, van Hasselt JGC: Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin Pharmacokinet 2019, 58(7): 835-857.
- Conforti F, Pala L, Pagan E, Corti C, Bagnardi V, Queirolo P, Catania C, De Pas T, Giaccone G: Sex-based differences in response to anti-PD-1 or PD-L1 treatment in patients with non-small-cell lung cancer expressing high PD-L1 levels. A systematic review and meta-analysis of randomized clinical trials. ESMO Open 2021, 6(5): 100251.
- Ozturk N, Ozturk D, Pala-Kara Z, Kaptan E, Sancar-Bas S, Ozsoy N, Cinar S, Deniz G, Li XM, Giacchetti S et al: The immune system as a chronotoxicity target of the anticancer mTOR inhibitor everolimus. Chronobiol Int 2018, 35(5): 705-718.
- Otasowie CO, Tanner R, Ray DW, Austyn JM, Coventry BJ: Chronovaccination: Harnessing circadian rhythms to optimize immunisation strategies. Front Immunol 2022, 13: 977525.
- Zhang H, Liu Y, Liu D, Zeng Q, Li L, Zhou Q, Li M, Mei J, Yang N: Time of day influences immune response to an inactivated vaccine against SARS-CoV-2. Cell Res 2021, 31(11): 1215-1217.
- Fontova P, Colom H, Rigo-Bonnin R, van Merendonk LN, Vidal-Alabró A, Montero N, Melilli E, Meneghini M, Manonelles A, Cruzado JM et al: Influence of the Circadian Timing System on Tacrolimus Pharmacokinetics and Pharmacodynamics After Kidney Transplantation. Front Pharmacol 2021, 12: 636048.
- Satoh S, Tada H, Murakami M, Tsuchiya N, Li Z, Numakura K, Saito M, Inoue T, Miura M, Hayase Y et al: Circadian pharmacokinetics of mycophenolic Acid and implication of genetic polymorphisms for early clinical events in renal transplant recipients. Transplantation 2006, 82(4): 486-493.
- Levi F, Le Louarn C, Reinberg A: Timing optimizes sustained-release indomethacin treatment of osteoarthritis. Clin Pharmacol Ther 1985, 37(1): 77-84.
- Arvidson NG, Gudbjörnsson B, Larsson A, Hällgren R: The timing of glucocorticoid administration in rheumatoid arthritis. Ann Rheum Dis 1997, 56(1): 27-31.
- Buttgereit F, Doering G, Schaeffler A, Witte S, Sierakowski S, Gromnica-Ihle E, Jeka S, Krueger K, Szechinski J, Alten R: Efficacy of modified-release versus standard prednisone to reduce duration of morning stiffness of the joints in rheumatoid arthritis (CAPRA-1): a double-blind, randomised controlled trial. Lancet 2008, 371(9608): 205-214.
- Buttgereit F, Mehta D, Kirwan J, Szechinski J, Boers M, Alten RE, Supronik J, Szombati I, Romer U, Witte S et al: Low-dose prednisone chronotherapy for rheumatoid arthritis: a randomised clinical trial (CAPRA-2). Ann Rheum Dis 2013, 72(2): 204-210.
- To H, Yoshimatsu H, Tomonari M, Ida H, Tsurumoto T, Tsuji Y, Sonemoto E, Shimasaki N, Koyanagi S, Sasaki H et al: Methotrexate chronotherapy is effective against rheumatoid arthritis. Chronobiol Int 2011, 28(3): 267-274.
- Sion B, Bégou M: Can chronopharmacology improve the therapeutic management of neurological diseases? Fundam Clin Pharmacol 2021, 35(3): 564-581.
- Klerman EB, Brager A: Keeping an eye on circadian time in clinical research and medicine. Clin Transl Med 2022, 12(12): e1131.
- Roenneberg T, Pilz LK, Zerbini G: Chronotype and Social Jetlag: A (Self-) Critical Review. Biology (Basel) 2019, 8(3): 54.
- Bailey M, Silver R: Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol 2014, 35(1): 111-139.
- Kennaway DJ: The dim light melatonin onset across ages, methodologies, and sex and its relationship with morningness/eveningness. Sleep 2023, 46(5): zsad033.
- Lévi F, Komarzynski S: Tele-Monitoring of Cancer Patients' Rhythms during Daily Life Identifies Actionable Determinants of Circadian and Sleep Disruption. Cancers (Basel) 2020, 12(7): 1938.
- Wittenbrink N, Ananthasubramaniam B, Münch M, Koller B, Maier B, Weschke C, Bes F, de Zeeuw J, Nowozin C, Wahnschaffe A et al: High-accuracy determination of internal circadian time from a single blood sample. J Clin Invest 2018, 128(9): 3826-3839.
- Vlachou D, Veretennikova M, Usselmann L, Vasilyev V, Ott S, Bjarnason GA, Dallmann R: TimeTeller: A tool to probe the circadian clock as a multigene dynamical system. PLoS Comput Biol 2024, 20(2): e1011779.
- Komarzynski S, Huang Q: Relevance of a Mobile Internet Platform for Capturing Inter- and Intrasubject Variabilities in Circadian Coordination During Daily Routine: Pilot Study. J Med Internet Res 2018, 20(6): e204.
- Ribeiro RFN, Cavadas C, Silva MMC: Small-molecule modulators of the circadian clock: Pharmacological potentials in circadian-related diseases. Drug Discov Today 2021, 26(7): 1620-1641.
- Hirota T, Lee JW, St John PC, Sawa M, Iwaisako K, Noguchi T, Pongsawakul PY, Sonntag T, Welsh DK, Brenner DA et al: Identification of small molecule activators of cryptochrome. Science 2012, 337(6098): 1094-1097.
- Dong Z, Zhang G, Qu M, Gimple RC, Wu Q, Qiu Z, Prager BC, Wang X, Kim LJY, Morton AR et al: Targeting Glioblastoma Stem Cells through Disruption of the Circadian Clock. Cancer Discov 2019, 9(11): 1556-1573.
- Humphries PS, Bersot R, Kincaid J, Mabery E, McCluskie K, Park T, Renner T, Riegler E, Steinfeld T, Turtle ED et al: Carbazole-containing amides and ureas: Discovery of cryptochrome modulators as antihyperglycemic agents. Bioorg Med Chem Lett 2018, 28(3): 293-297.
- Gul S, Rahim F, Isin S, Yilmaz F, Ozturk N, Turkay M, Kavakli IH: Structure-based design and classifications of small molecules regulating the circadian rhythm period. Sci Rep 2021, 11(1): 18510.
- Gul S, Akyel YK: Discovery of a small molecule that selectively destabilizes Cryptochrome 1 and enhances life span in p53 knockout mice. Nat Commun 2022, 13(1): 6742.
- Doruk YU, Yarparvar D, Akyel YK, Gul S, Taskin AC, Yilmaz F, Baris I, Ozturk N, Türkay M, Ozturk N et al: A CLOCK-binding small molecule disrupts the interaction between CLOCK and BMAL1 and enhances circadian rhythm amplitude. J Biol Chem 2020, 295(11): 3518-3531.
- He B, Nohara K, Park N, Park YS, Guillory B, Zhao Z, Garcia JM, Koike N, Lee CC, Takahashi JS et al: The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab 2016, 23(4): 610-621.
- Solt LA, Wang Y, Banerjee S, Hughes T, Kojetin DJ, Lundasen T, Shin Y, Liu J, Cameron MD, Noel R et al: Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485(7396): 62-68.
- Sulli G, Rommel A, Wang X, Kolar MJ, Puca F, Saghatelian A, Plikus MV, Verma IM, Panda S: Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553(7688): 351-355.
- Lee Y, Lahens NF: G1/S cell cycle regulators mediate effects of circadian dysregulation on tumor growth and provide targets for timed anticancer treatment. PLoS Biol 2019, 17(4): e3000228.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript