Review Article | Open Access
New developments in the nanocarrier-based drug delivery system for the treatment of breast cancer
Reem Al Yahyai1, Jamilah Al Kalbani1
1Department and Building of Biotechnology, Sultan Qaboos University, Al-Khod District, Muscat Governorate, Sultanate of Oman.
Correspondence: Reem Al Yahyai (Department and Building of Biotechnology, Sultan Qaboos University, Al-Khod District, Muscat Governorate, Sultanate of Oman; E-mail: r.alyahyai@gmail.com).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2025, 5: 33-43. https://doi.org/10.32948/ajpt.2025.03.10
Received: 15 Feb 2025 | Accepted: 16 Mar 2025 | Published online: 24 Mar 2025
Key words breast cancer, nanomaterials, nanoparticles, liposomes, drug delivery system
Nanoparticles (NPs) are thought to be one of the possible delivery systems which could take care of clinical application issues. NPs can generally be defined as colloidal particles with a size range of 10-1000 nm [6]. They may increase target selectivity, stability, and bioavailability, thus augmenting therapeutic approaches. All these advantages lead to minimized side effects while taking care of the drawbacks of classical therapeutic approaches [7]. Additionally, by improving the pharmacokinetics of drugs, prolonging half-life, and increasing drug solubility [8]. NPs can advantage drug delivery. Unfortunately, NPs as a drug carrier system have more advantages than disadvantages. By solubilizing hydrophobic substances in an aqueous solution, they are able to increase the drug solubility, maintain its stability, and extend blood circulation time. Targeted delivery of the drug with NP further minimizes side effects and develops countermeasures for drug resistance. All things considered, NPs show stability in vivo and offer an effective way to deliver medications to the target region [9].
Because of their improved drug delivery and therapeutic effects on a variety of cancer types, drug-loaded NPs are currently thought to be very promising for effective cancer treatment [10]. Polymeric nanomedicines are another name for polymeric nanocarriers, which encapsulate or conjugate anticancer medications. These nanocarriers come in a variety of shapes, such as dendrimers, vesicles, micelles, nanospheres, nanogels, and polymer–drug conjugates [11]. Their capacity to specifically target tumor cells and co-deliver numerous therapeutic medicines is what is driving their increasing popularity [12]. These nanoparticle systems can transport a wide variety of therapeutic molecules, including cytotoxic medicines, small interference RNA (siRNA), chemosensitizers, and antiangiogenic agents [13]. The distinct pathophysiology of cancer cells causes NPs to increase permeability and the retention effect. Increased intracellular medication concentrations result from NPs building up inside tumor cells without P-glycoprotein recognizing them. With minimal harm to healthy peripheral tissues, this capability allows NPs to deliver chemotherapy directly to tumor cells at the ideal time, guaranteeing excellent efficacy [14]. In this context, a multitargeting system (MTS) has drawn a lot of interest. By adding several functional ligands to the NP surface that can bind to various cell surface receptors, this tactic improves the multivalent contacts between the NPs and the target cells, increasing cellular recognition and uptake. Drug efficacy is further enhanced by other strategies, such as adding pH-sensitive groups [15]. Numerous multifunctional NPs are being investigated as NPs continue to improve cancer therapy methods.
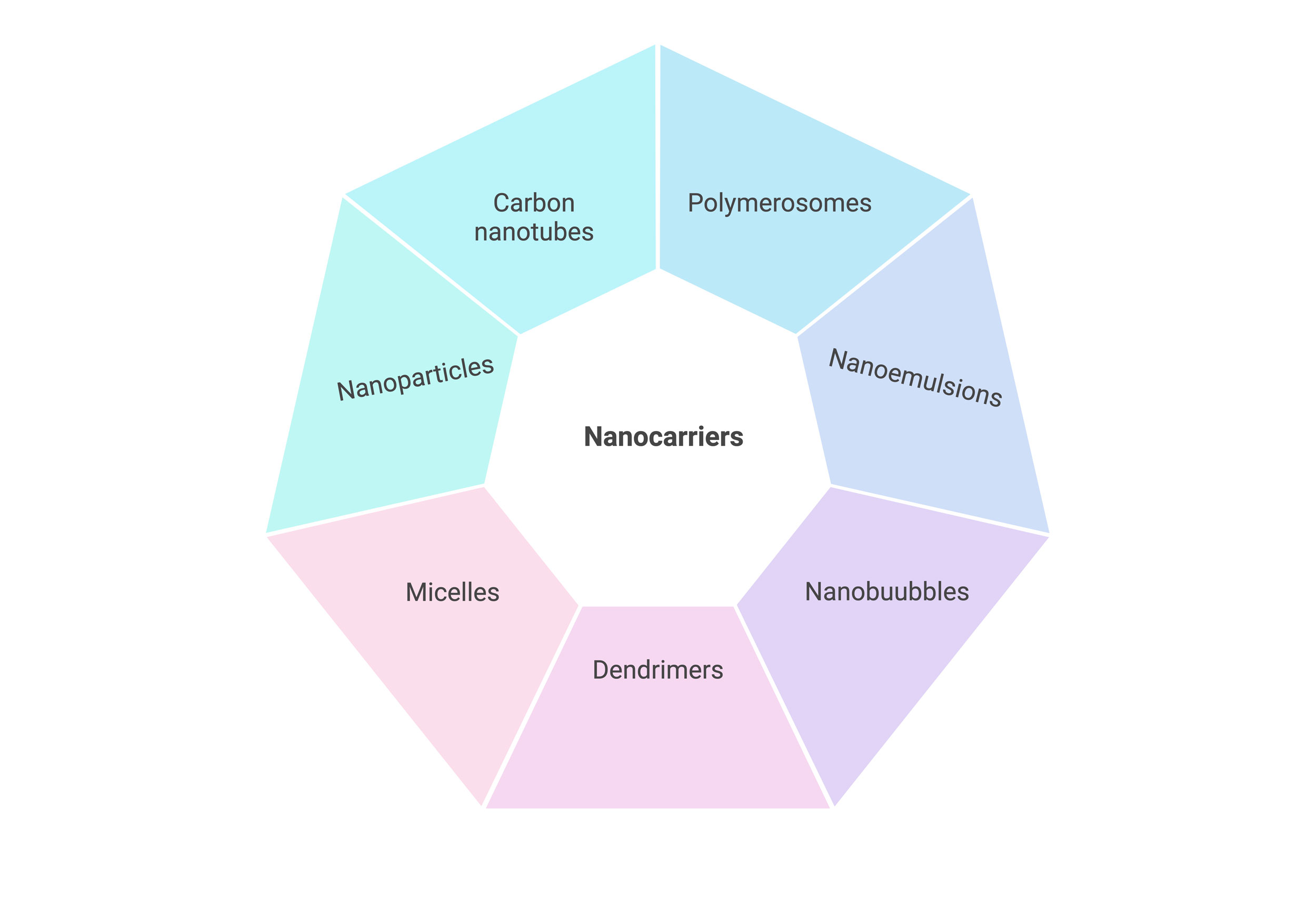 Figure 1. Different classifications of nanocarriers on the basis of structure and morphology.
Figure 1. Different classifications of nanocarriers on the basis of structure and morphology.
One possible way to address issues related to BC is using nanotechnology. Numerous scientists are investigating different drug delivery methods based on nanotechnology and how they work to treat breast cancer. BC can be detected using a variety of nanoparticle forms, although the most widely used ones are carbon nanorods (e.g., gold nanorods [19]), nanowires (e.g., gold nanowires [20]), and nanobarcodes. In nanotechnology, semiconductor quantum dots (QDs) are an interesting new development. Compared to fluorescent proteins and chemical dyes, these nanoscale light-emitting particles have benefits. Semiconductor QDs are perfect for cellular and in vivo biomolecular imaging because of their special electrical and optical characteristics [21]. Yu et al. created blue light-emitting QDs based on cadmium oxide and selenium with a diameter of around 2 nanometers and red light-emitting QDs with a diameter of 7 nanometers [22]. High resolution and sensitivity have been established using combined optical and X-ray imaging, which enables the detection of both small, aberrant tumor daughter cells and big BC tumors. Chemotherapy NPs can be administered by active or passive routes. Through the use of biomarkers and probes based on NPs, nanotechnology also contributes to the diagnosis of molecular cancer. Individual NPs can be attached to several ligands, resulting in a multivalent effect that improves binding affinity and specificity, making them useful diagnostic tools.
Cytotoxic medications must be completely absorbed by cancer cells in order to treat the disease effectively. Overcoming chemotherapeutic resistance requires increasing medication absorption [26]. Drug efflux mediated by transmembrane pumps is one of the main mechanisms underlying treatment resistance. In order to overcome multidrug resistance to chemotherapeutic medications, researchers have created acid-grafted poly(b-amino ester) NPs for encapsulating cleavage protein B. In lower pH levels, the pH-sensitive releasing mechanism of these NPs improves drug release. Importantly, by blocking P-glycoprotein (P-gp) expression and interfering with the energy source for drug efflux, they can also reverse multidrug resistance, offering a novel approach to treating BC [27]. Based on star-shaped polyester (FA-TRI-CL), Guo's group created folic acid-modified NPs ((DOX + CUR)-FA-NPs) that efficiently target P-gp to overcome resistance. One transmembrane transporter that contributes to chemotherapy resistance is P-gp, which releases medications from cells. (DOX + CUR)-FA-NPs increase drug accumulation in tumor cells and reduce resistance by blocking P-gp, which stops resistant cells from removing the medications [28]. Additionally, D-a-tocopherol polyethylene glycol 1000 succinate-resveratrol solid lipid NPs (TPGS-Res-SLNs) were developed by researchers, and they were successful in lowering the expression of proteins linked to multidrug resistance, such as GRP and BCRP. This decrease increases the concentration and duration of effect of chemotherapeutic medicines by reducing their excretion and inactivation within cells. Additionally, TPGS-Res-SLNs improved the effectiveness of chemotherapy by inhibiting the EMT in cancer cells, which decreased their capacity to invade and spread [29].
The main phases of hormone-independent BC carcinogenesis, which include angiogenesis, metastasis, cell proliferation, and survival, are all significantly influenced by cell signaling [30]. An overview of the role these signaling pathways play in chemotherapy resistance in BC is given in this section. It has been demonstrated that PI3K/AKT/mTOR signaling pathway dysregulation facilitates cancer spread and increases multidrug resistance. A pH-sensitive nanocomplex was created by Yin et al. to co-deliver siRNA and paclitaxel (PTX) to metastatic breast cancer. In 4T1 metastatic BC cells, the siRNA targets and inhibits Akt expression. Micelle/siAkt nanocomplexes (PMA) loaded with PTX effectively downregulated P-gp, boosted Caspase-3 production, and knocked down the Akt gene in these cells. In addition to showing encouraging in vitro outcomes, PMA proved to be safe and effective in vivo. PMA reduced lung metastases of BC by 96.8% and inhibited tumors by 94.1% in 4T1 tumor-bearing mice. Additionally, the PMA nanocomplex did not generate lesions in normal tissues and showed very low toxicity [31]. TNBC cells commonly overexpress Notch1 receptors and underexpress miR-34a microRNA, according to Guney et al. developed N1-34a-NPs by encapsulating miR-34a mimics in poly NPs in order to address this. In order to disrupt Notch signaling and the cascade that goes along with it, these NPs were functionalized to target the overexpressed Notch1 receptors on the surface of TNBC cells. Their results demonstrated that N1-34a-NPs efficiently controlled Notch signaling and its downstream targets in TNBC cells, leading to decreased migration and proliferation as well as cell senescence [32].
The present research has established the novel combined therapeutic approach involving both doxorubicin (DOX) and its dietary component, indole 3,3'-diindolylmethane (DIM). In this introduction of the two agents by loading them onto exosome-encapsulated mesoporous silica NPs (e-DDMSNP), DIM and DOX were co-delivered to the cancer stem cell (CSC). This approach would increase specificity, stability, and homing potential towards the CSC niche for both DIM and DOX in vitro and in vivo. Thus, our innovative exosome nano-preparation holds good promise to target CSCs following the EMT process [33]. Targeting the EMT process using the inhibition by SKN, SKN@FPD NM is a nanodrug delivery method for metastasis risk reduction of BC cells. The combination further enhances tumor cell lethality while decreasing the probability of drug resistance and treatment failure [34]. In summary, nanodrug delivery devices hold promise for treatment resistance in BC. There are thus strong chances for these systems to be effective in conquering drug resistance in various types of cancer. Programmed DNA self-assembly of nanosystems loaded with Rab26 siRNA into NPs for drug resistance in lung cancer was developed by researchers [35]. In the same way, RAW-PANP can facilitate PTX and miRNA delivery, proposing a novel strategy to treat PTX-resistant TNBC. Beyond this, RAW-PANP can also serve as a competent platform for targeted therapy in TNBC [36].
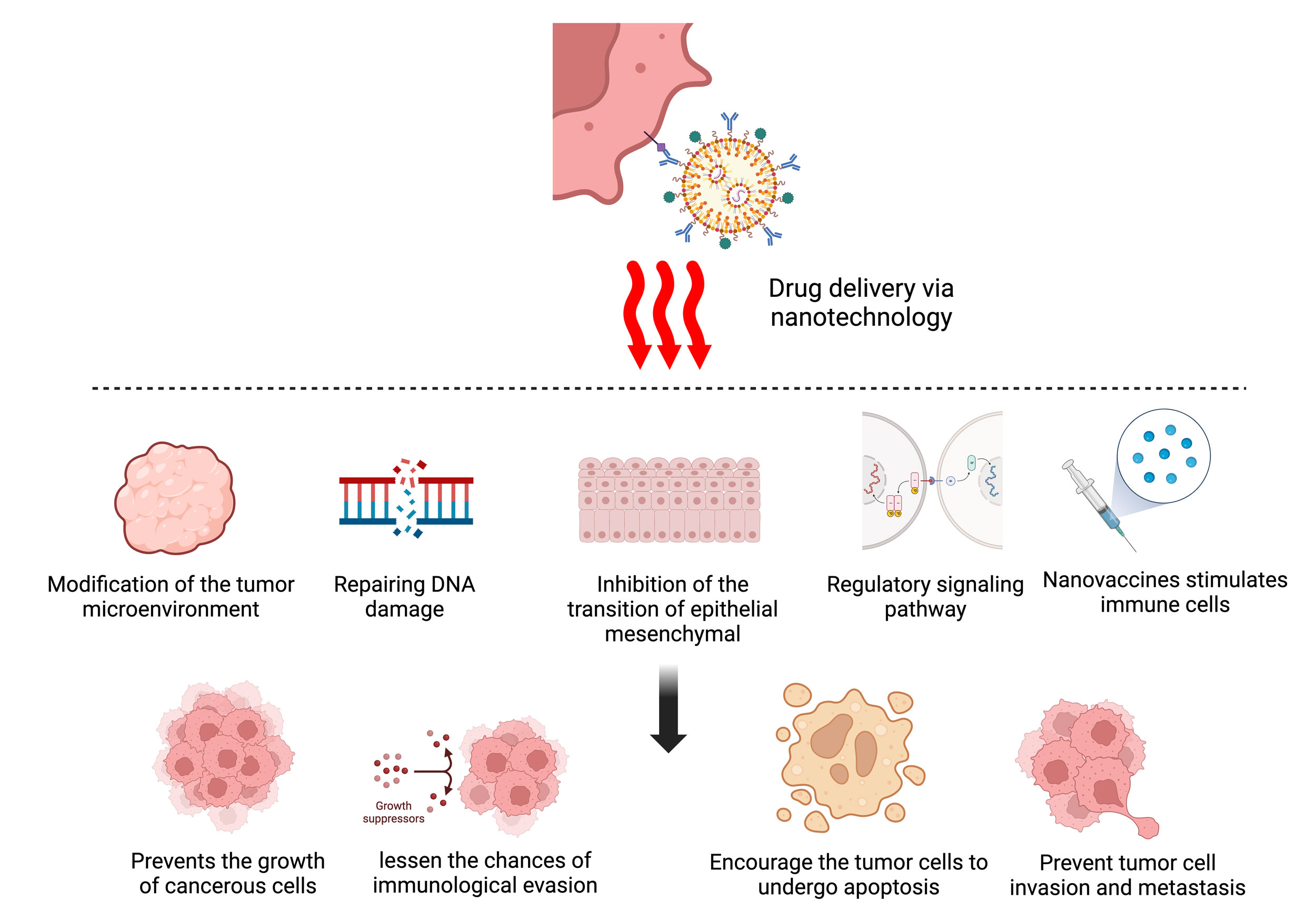 Figure 2. Novel nanodrug delivery techniques utilize a range of mechanisms to target breast cancer: immune system activation, apoptotic induction, tumor microenvironment manipulation, DNA damage repair, and metastasis suppression. Collectively, these mechanisms inhibit tumor growth, enhance treatment efficacy, and reduce the chances of drug resistance, laying the groundwork for advanced precision oncology options.
Figure 2. Novel nanodrug delivery techniques utilize a range of mechanisms to target breast cancer: immune system activation, apoptotic induction, tumor microenvironment manipulation, DNA damage repair, and metastasis suppression. Collectively, these mechanisms inhibit tumor growth, enhance treatment efficacy, and reduce the chances of drug resistance, laying the groundwork for advanced precision oncology options.
Liposomes
The closed spherical vesicle structure of liposomes, which are nanocarriers made of a phospholipid bilayer, sets them apart. Liposomes can fuse with cell membranes because of their makeup, which is similar to biological membranes. This allows for the targeted release of medications into cells. They can improve drug loading, ensure sustained, controlled release of the drug, and encapsulate both hydrophilic and hydrophobic drugs [41]. In their discussion of liposomes' benefits for intracellular drug delivery, Boratto et al. emphasized how well they would work as carriers for BC treatment. They demonstrated improved cellular drug delivery, increased tumor accumulation, and a regulated release of DOX with their pH-sensitive liposome formulation (pHSL-TS-DOX). Additionally, pHSL-TS-DOX therapy caused cell cycle arrest, especially in the G1 phase, which could aid in reducing the growth of tumor cells [42]. Furthermore, Badr-Eldin et al.'s APA-functionalized liposomes (EGA-EML-APA) showed enhanced cytotoxicity against human BC cells. This was accomplished by causing MCF-7 cells to arrest in the G2/M and S phases, as well as by upregulating p53, bax, and casp3, downregulating bcl2, lowering NF-kB activity, increasing TNFa expression, and causing notable apoptotic events [43]. KLA-modified liposomes co-loaded with paclitaxel and 5-fluorouracil (KLA-5-FU/PTX Lps) were produced by Chen et al. and shown increased cytotoxicity against MDA-MB-231 cells. According to their suggested anti-tumor mechanism, the KLA peptide selectively targets mitochondria and facilitates liposome uptake by tumor cells. This causes the mitochondrial membrane to be disrupted, membrane potential to be lost, cytochrome C to be released, caspase-3 to be upregulated, and the apoptotic pathway to be activated in tumors. As a result, KLA-5-FU/PTX Lps offers a potentially effective treatment approach for TNBC [44]. When taken as a whole, these results highlight the substantial therapeutic potential of functionalized liposomes for breast cancer (Figure 3).
Solid lipid nanoparticles
Solid lipid nanoparticles (SLNs) are constructed of biocompatible physiological lipid components, making them extremely compatible with biological systems. To improve blood circulation and tumor selectivity, Granja et al. created SLNs that contain the anticancer medication mitoxantrone and functionalized them with 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[folate (polyethylene glycol)] (DSPE-PEG-FA) ligands. By means of processes including clathrin-mediated endocytosis and macropinocytosis, this functionalization enhances cellular uptake while lowering systemic side effects [45]. In order to increase drug solubility and bioavailability and treat tumor cells more successfully, Aly et al. created CS/Lf/PTS-SLNs. By suppressing vascular endothelial growth factor, downregulating cyclin D1, and upregulating caspase-3 and BAX, these SLNs improve the effectiveness of medications [46]. Da Rocha et al.'s SLN-DTX promotes cell accumulation in the G2-M phase, boosts drug uptake in cells, and triggers apoptosis, all of which increase cytotoxicity. Figure 1 illustrates the ways in which SLN-DTX suppresses tumor development, limits lung metastasis, decreases cell proliferation, enhances tumor cell death, and lowers BCL-2 expression [47]. According to Pindiprolu et al., PBA-Niclo-SLN reduces tumor recurrence in TNBC by inducing G0/G1 phase arrest and death, targeting STAT3, CD44/CD24 TNBC stem cell subsets, and altering markers of EMT [48]. According to these findings, solid lipid NPs have a lot of potential for use as therapeutic agents to treat BC and stop its spread and recurrence.
Dendrimers
Dendrimers NPs which usually range in size from 1 to 100 nm, are composed of three primary parts: a central core, repeating branching units, and outside surface functional groups [49]. Since the core of dendrimers is often hydrophobic, they are especially useful for increasing the solubility of hydrophobic medications [50]. Higher drug content and more effective targeted drug delivery are made possible by the diverse modification options provided by the branched architecture of dendrimers [51]. Additionally, medications, targeted ligands, and imaging agents can be coupled with or significantly alter the outer surface functional group [52]. Poly (L-lysine) (PLL) dendrimers, polypropylene imine (PPI) dendrimers, polyamidoamine (PAMAM) dendrimers, and PAMAM-organosilicon dendrimers (PAMAMOS) are often employed dendrimers in cancer treatment [49]. Due to so many of these odd characteristics the dendrimers are really apt for drug-delivery in cancer therapies. For instance, Guo et al. (2019) [53] developed a novel PAMAM dendrimer nanoparticle that had been modified with hyaluronic acid (HA) to deliver DOX and cisplatin in a combined manner (HA@PAMAM-Pt-Dox). The findings revealed that HA@PAMAM-Pt-Dox effectively wiped out BC cells and significantly enhanced the therapeutic effect of DOX and cisplatin.
Polymeric micelles
Polymer micelles are considered nanocarriers as they self-assemble hydrophilic shells with their hydrophobic cores [54]. Hydrophobic cores can be constructed from materials like PCL, PLA, or PLGA, whereas hydrophilic shells comprise materials like PEG, PGA, or even PEI [55]. Polymer micelles function with an average particle size of 5 to 100 nm, making them the best drug carriers penetrating tumor vasculature [56]. In addition, a hydrophobic core may hold various hydrophobic drugs in these micelles, which makes it very beneficial for the delivery of anticancer drugs like paclitaxel (PTX), which has low solubility in water. Peng et al. (2019) developed worm-like nanocrystal micelles from Herceptin-conjugated PTX-loaded PCL-PEG to treat HER2-positive breast cancer. Their results showed that the PTX-loaded micelles provided accurate targeting of HER2+ tumor cells while being safe for normal tissues and stable in both the tumor microenvironment and circulation [57].
Polymeric nanoparticles
Polymeric nanoparticles (P-NPs) can encapsulate or conjugate chemotherapeutic medications for distribution; they are made from biodegradable and biocompatible raw materials, usually polyesters (Table 2) [58]. However, regulating elements such the polymers' molecular weight, polydispersity, and stereoregularity is a significant issue in the production of P-NPs [59]. Nowadays, ring-opening polymerization of lactone or lactide monomers, assisted by organometallic catalysts, is a typical method for creating polyesters like polycaprolactone (PCL) and polylactide (PLA) [59]. Additionally, naturally existing biocompatible polymers with functional groups like ether, amide, and ester can be used to create P-NPs [59]. FDA-approved biocompatible polymers for drug delivery include PCL, PLA, and poly(lactic-co-glycolic acid) (PLGA); additional polymers used in drug delivery applications include polyethylene glycol (PEG), chitosan (CS), and hyaluronic acid (HA) [50]. Moreover, P-NPs can be designed to target particular areas and regulate drug release by changing the surface of these polymers or their characteristics with particular ligands, improving bioavailability and therapeutic efficacy [60].
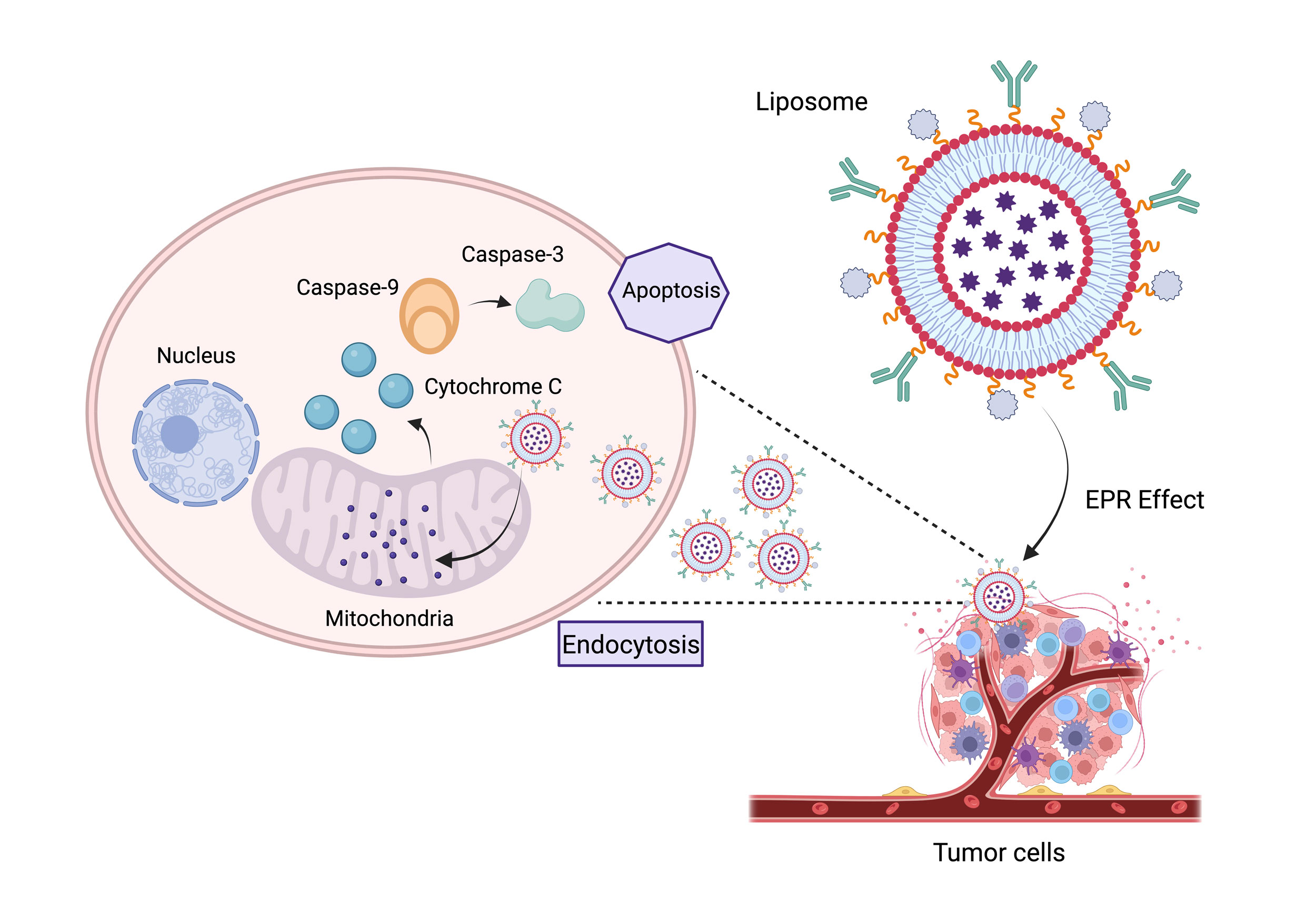 Figure 3. An improved liposomal nanocarrier technology designed for targeted mitochondrial distribution in breast cancer treatment is depicted in this figure. By using an EPR-driven uptake mechanism, the technology enables tumor cells to be selectively endocytosed. The nanocarriers deliver a multi-drug payload during intracellular trafficking, which causes ROS production, mitochondrial depolarization, and cytochrome C-mediated death. The strategy offers a promising path for next-generation cancer therapeutics by increasing therapeutic efficacy while reducing off-target damage.
Figure 3. An improved liposomal nanocarrier technology designed for targeted mitochondrial distribution in breast cancer treatment is depicted in this figure. By using an EPR-driven uptake mechanism, the technology enables tumor cells to be selectively endocytosed. The nanocarriers deliver a multi-drug payload during intracellular trafficking, which causes ROS production, mitochondrial depolarization, and cytochrome C-mediated death. The strategy offers a promising path for next-generation cancer therapeutics by increasing therapeutic efficacy while reducing off-target damage.
|
Table 1. Types of nanocarriers for drug delivery. |
|||
|
Nanomaterials |
Structure |
Features |
Reference |
|
Liposomes |
Self-assembled closed colloidal structures are formed by lipid bilayer memberane |
Bioabsorbable, extended period of circulation, bipolar |
[81] |
|
Polymeric micelles |
Amphiphilic block copolymer is assembled to generate a hydrophilic shell and a hydrophobic core |
Effective medication delivery method for hydrophilic substances, bioabsorbable, potential targeting, adaptive modification |
[82] |
|
Dendrimers |
A synthetic polymer that forms a nanoscale branching structure with regular patterns and repeating units |
Uniformity in branch length, size, and shape, altered biodistribution and pharmacokinetics, targeting, loading, and surface area are all increased |
[83] |
|
Carbon nanotubes
|
Carbon cylindrical structure formed by the benzoene ring |
Water soluble, chemically modified, multifunctional, and bioabsorbable |
N/A |
|
Nanorods |
Rod-shaped structures made of metals or semiconducting materials |
Increased surface area, biocompatibility, targeted tumors, and efficient loading |
[84] |
|
Table 2. Recent polymeric nanoparticles explored for breast cancer therapeutics. |
||||
|
Polymer |
Drug |
Preparation technique |
Outcomes |
Reference |
|
Polycaprolactone, PEG, stearic acid |
Anastrozole |
Solvent evaporation in direct emulsification |
Increased effectiveness of treatment |
[85] |
|
PLGA, Labrafil M2125 CS oil |
Baicalin |
Nano-precipitation |
Effective in enhancing anticancer potential |
[86] |
|
Bovine serum albumin |
Curcumin |
Desolvation |
Treatment of breast cancer |
[87] |
|
PLA |
Calcitriol |
Nano-precipitation |
Prolonged and sustained anticancer action; increased effectiveness of treatment |
[88] |
|
Bovine serum albumin, polyethylene glycol |
Curcumin |
Desolvation |
Improved efficacy for breast cancer |
[89] |
|
Triethylamine, PLGA |
Docetaxel |
Solvent evaporation |
Demonstrated a prolonged release, high affinity, and extreme sensitivity for cancer cells |
[90] |
|
Monophosphoryl lipid A |
Doxorubicin |
Conjugation (ROS switchable nano- platform) |
Reduced systemic toxicity and effective tumor targeting |
[91] |
|
PLGA |
Rapamycin |
Single emulsion solvent evaporation |
Drug targeted to epidermal growth factor receptor; efficient tumor selectivity |
[92] |
|
Pluronic F127 |
Paclitaxel, lapatinib |
Thin-film hydration |
Improved treatment for metastatic breast cancer |
[93] |
|
PLGA |
Simvastatin |
Spray drying with nanotechnology |
Treatment of solid tumor |
[94] |
|
Alginate |
Paclitaxel |
Nano-emulsification polymer cross- linking |
Improved breast cancer anticancer effects |
[95] |
One important illustration of the successful application of NPs in cancer treatment is Abraxane, an albumin-bound form of paclitaxel [62]. Through the increased permeability and retention (EPR) effect, this formulation passively targets tumor tissues by utilizing albumin's inherent characteristics. Because tumor areas frequently have impaired lymphatic drainage and vascular, the EPR effect is crucial for the buildup of therapeutic drugs in the tumor microenvironment. By enabling a greater concentration of the medication at the tumor site, passive targeting enhances treatment efficacy while reducing the harmful side effects frequently associated with systemic drug delivery [62]. Active targeting techniques are leveraged by newer nanoparticle formulations like ELU001 and CALAA-01, which are aimed to enhance the specificity of drug delivery by targeting molecules that are overexpressed on cancer cells [63, 64].
Passive targeting
The enhanced permeability and retention (EPR) effect is common passive targeting in cancer therapy. EPR works to increase delivery efficacy through unique tumor pathophysiological characteristics. The phenomenon can be seen in tumor tissues, where the newly formed blood vessels are even more permeable due to large fenestrations between endothelial cells when compared to that in normal tissues; this allows greater accumulation of the NPs in the tumor microenvironment [65]. The EPR effect is especially enhanced for about 10 to 100 nm-sized NPs because they won't be rapidly cleared by kidneys and will therefore be able to deposit greater payloads of the therapeutic agents into the tumor with lesser systemic toxicity [65].
The first FDA-approved medication based on NPs, Doxil®, contains liposomal DOX, which is intended to use the EPR effect to deliver medication specifically to BC patients. According to clinical research, Doxil® preserves its anticancer effectiveness while improving patient outcomes by lowering the cardiotoxicity linked to free DOX [66]. By altering the surface of NPs with hydrophilic polymers such as PEG, for example, it has been discovered that this reduces protein adsorption, which prolongs circulation time and increases tumor accumulation through the EPR effect. This strategy was successfully used in the formulation of Abraxane®, which exhibits improved tumor targeting and has been approved for the treatment of metastatic BC [62]. More sophisticated nanoparticle systems have been developed recently that further enhance the EPR effect.
More recently, it has been established that the EPR effect is impacted by the behavior of NPs in the tumor microenvironment, which is influenced by tumor pH, hypoxia, and enzyme activity. According to Chen et al., the tumor's acidic and hypoxic circumstances can be used to create pH-sensitive NPs that, when the pH drops, release their therapeutic payload, improving drug release precisely at the tumor site and hence improving therapeutic results [67]. Furthermore, current studies are aimed at improving nanoparticle design to overcome the difficulties posed by the EPR effect, including variations in vascular permeability and shifting interstitial pressures that may prevent nanoparticle penetration in specific malignancies. Strategies like as shape engineering, size modulation, and surface charge optimization are being researched to improve the therapeutic efficacy of passively targeted NPs in order to overcome these obstacles [65].
Passive targeting in conjunction with other therapeutic approaches has also attracted a lot of interest. It has demonstrated potential in increasing nanoparticle accumulation in tumors, for example, by combining it with hyperthermia, a condition in which localized heat momentarily improves vascular permeability at the tumor location. This enhances the therapeutic benefit without aggravating systemic negative effects. The study conducted by Basu et al. demonstrated that this synergistic mechanism indeed enhanced drug delivery to breast malignant tumors by combining magnetic NPs with localized hyperthermia [68]. The advances in the technologies that allow real-time monitoring of the efficiency of tumor targeting and nanoparticle distribution are further enhancing the possibility of passive targeting. The development of NPs with imaging capabilities that are trackable using imaging approaches such as magnetic resonance imaging (MRI), PET, or fluorescence imaging can serve as an illustration of this advancement. This double work permits the tuning of individual therapy regimens, complementing the validation of nanoparticle accumulation at tumor sites. An effective mode of patient-tailored cancer therapy is provided by theranostics, a fascinating area of study that integrates therapeutic and diagnostics. For example, Chauhan et al. illustrated in a study of magnetic NPs in MRI and hyperthermia how the NPs could enhance drug delivery via the EPR effect while providing real-time assessment of therapeutic efficacy [69]. The combination of genetic engineering and nanotechnology is another interesting area of study for the treatment of cancer. For example, gene therapy can be used to target particular cancer cells, while sparing normal healthy tissues, by coupling it with such nanoparticle delivery technologies. Li et al. were able to deliver CRISPR-Cas9 systems targeting different oncogenes within BC cells with lipid-based NPs. Their results indicate that this may offer extremely effective and much less invasive alternatives to traditional radiotherapy and chemotherapy, thereby establishing a new gold standard in cancer treatment [70]. Combination therapy in which NPs are used to co-deliver several compounds at the same time has also shown much promise. Studies have shown that administering immunochemotherapeutics through NPs also greatly improved the therapeutic effect [71]. Maximization of distribution is then by enhancing the stability and circulation time of NPs. Encouraging results have come up with the latest innovations in coating material for NPs. New zwitterionic nanoparticle coating significantly reduced uptake by the mononuclear phagocyte system in an investigation by McMullen designed to prolong systemic circulation. This may enhance therapeutic index for anticancer drugs through higher concentrations of drugs at the tumor site [72].
Active targeting
Active targeting of drug delivery systems based on NPs for cancer therapy, requires molecules that can selectively identify and bind to receptors or biomarkers that are overexpressed on the surface of cancer cells. The objective of this approach is to improve the accuracy and potency of delivering the medicine to malignant sites. For instance, peptide or antibody-modified NPs that bind to transmembrane receptors associated with cancer are a means of targeting mainly cancer cells. This reduces side effects while at the same time enhancing the efficacy of anticancer treatments. Moreover, drug release from NPs can be regulated and sustained, hence eliminating the need for a regular dosing regimen. Furthermore, more sophisticated nanomedicine might be enabled to cross biological barriers, such as the blood-brain barrier, thus allowing targeted drug delivery with enhanced therapeutic effects [73].
Antibody fragments such as Fab, Fab', Fv, single-chain variable fragments (ScFv), and single-domain antibodies (or nanobodies) have been revealed recently to offer a novel dimension of opportunity as efficient targeting ligands on NPs [74]. The fragments derive benefits from easy genetic manipulation, economical manufacture, and highly specific targeting. Their reduced size gives them a distinct therapeutic advantage to make them user-friendly in multifunctional drug formulations and improves tissue penetration [75]. In addition, the results regarding the improvement of the specificity and efficacy of drug delivery through incorporating antibody fragments into whole nanoparticle systems have also been favorable. These conjugates have exposed much greater cytotoxicity and targeted efficacy against pancreatic cancer cells; therefore, they might also be relevant in treating BC [74].
This progress is demonstrated in a ground-breaking study by Marshall et al., which developed biomimetic, targeted theragnostic NPs for the treatment of breast cancer. The work used NPs modified with targeting ligands and coated with human red blood cell membranes, using biomimetic principles. These NPs showed a better capacity to target MCF-7 BC cells that express epithelial cell adhesion molecule (EpCAM) by encapsulating both chemotherapeutic drugs and imaging markers [76]. These NPs' dual purpose enabled accurate cancer cell imaging and targeted drug delivery, leveraging the special powers of nanoscale engineering to get beyond the drawbacks of conventional therapies. This strategy is a step toward personalized medicine, where tracking the growth of cancer can inform changes to treatment regimens for the best results. It also demonstrates the idea of theranostics, which is a blend of therapy and diagnostics [77]. This illustration shows how nanotechnology can support an all-encompassing approach to cancer detection and therapy.
One-of-a-kind unique excellence has been given to the field by Marshall et al. in their ground-breaking research involving the engineering of biomimetic and targeted theragnostic NPs dedicated to the treatment of breast cancer. These were developed according to biomimetic principles through the utilization of targeting ligand-modified NPs coated with human red blood cell membranes. These NPs targeted MCF-7 BC cells more efficiently as they were better than those not containing the encapsulated chemotherapeutics and imaging markers. Therefore, these NPs have made it easy to image the cancer cells and deliver the drugs targetedly, in nanoscale engineering that avoids some of the disadvantages of traditional therapies. This forms part of advances leading to personalized medicine where tracing the growth of cancer could determine timely changes in treatment regimens for the best possible outcomes. This is primarily the second aspect of what theranostics is all about; the amalgamation of therapy and diagnostics in one. This is an example of how nanotechnology could offer a synergistic contribution to cancer diagnostics and treatment efforts [78].
The creation of NPs that selectively target tumor-associated receptors is one recent advancement in this field. The application of ligands, such as antibodies, aptamers, or tiny molecules that attach to cancer-specific receptors, was a significant development in this area. For instance, customized NPs have been effectively used to target the HER2 receptor, which is overexpressed in around 30% of instances of BC [79]. Furthermore, new avenues for all-encompassing cancer treatment are being opened by the investigation of multifunctional NPs that combine targeted drug delivery with other therapeutic modalities, such as radiation or heat. Herea et al.'s noteworthy invention included magnetic NPs that were guided to the tumor site by means of external magnets. These NPs caused localized hyperthermia when exposed to alternating magnetic fields, which disrupted tumor cells and improved chemotherapeutic medication absorption, greatly increasing treatment outcomes [80].
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
Reem Al Yahyai contributed to draft, critical revision of the article, table making, and final submission; Jamilah Al Kalbani revised the manuscript and drew figures.
Competing interests
The authors declare no competing interests.
- Tosca EM, Bartolucci R, Magni P: Application of artificial neural networks to predict the intrinsic solubility of drug-like molecules. Pharmaceutics 2021, 13(7): 1101.
- Lauersen NH, Birnbaum SJ: Water intoxication associated with oxytocin administration during saline-induced abortion. Am J Obstet Gynecol 1975, 121(1): 2-6.
- Fontana F, Carollo E, Melling GE, Carter DR: Extracellular vesicles: emerging modulators of cancer drug resistance. Cancers 2021, 13(4): 749.
- Deng Y, Huang R, Huang S, Xiong M: Nanoparticles enable efficient delivery of antimicrobial peptides for the treatment of deep infections. BIO Integration 2021, 2(2): 50-56.
- Kim KR, Kim DR, Lee T, Yhee JY, Kim BS, Kwon IC, Ahn DR: Drug delivery by a self-assembled DNA tetrahedron for overcoming drug resistance in breast cancer cells. Chem Commun 2013, 49(20): 2010-2012.
- Zheng C, Li M, Ding J: Challenges and opportunities of nanomedicines in clinical translation. BIO Integration 2021, 2(2): 57-60.
- Ndebele RT, Yao Q, Shi YN, Zhai YY, Xu HL, Lu CT, Zhao YZ: Progress in the application of nano-and micro-based drug delivery systems in pulmonary drug delivery. BIO Integration 2022, 3(2): 71-83.
- Palacio-Castañeda V, Oude Egberink R, Sait A, Andrée L, Sala BM, Hassani Besheli N, Oosterwijk E, Nilvebrant J, Leeuwenburgh SC, Brock R et al: Mimicking the biology of engineered protein and mRNA nanoparticle delivery using a versatile microfluidic platform. Pharmaceutics 2021, 13(11): 1944.
- Li Z, Xiao C, Yong T, Li Z, Gan L, Yang X: Influence of nanomedicine mechanical properties on tumor targeting delivery. Chem Soc Rev 2020, 49(8): 2273-2290.
- Truong NP, Whittaker MR, Mak CW, Davis TP: The importance of nanoparticle shape in cancer drug delivery. Expert Opin Drug Deliv 2015, 12(1): 129-142.
- Liu Y, Feng L, Liu T, Zhang L, Yao Y, Yu D, Wang L, Zhang N: Multifunctional pH-sensitive polymeric nanoparticles for theranostics evaluated experimentally in cancer. Nanoscale 2014, 6(6): 3231-3242.
- Liu J, Xu M, Yuan Z: Immunoscore guided cold tumors to acquire “temperature” through integrating physicochemical and biological methods. BIO Integration 2020, 1(1): 6-14.
- Lammers T, Ferrari M: The success of nanomedicine. Nano Today 2020, 31: 100853.
- Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P: Nanoparticle-based immunotherapy for cancer. ACS Nano 2015, 9(1): 16-30.
- Asad MI, Khan D, Rehman AU, Elaissari A, Ahmed N: Development and in vitro/in vivo evaluation of pH-sensitive polymeric nanoparticles loaded hydrogel for the management of psoriasis. Nanomaterials 2021, 11(12): 3433.
- Brinton LA, Sherman ME, Carreon JD, Anderson WF: Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst 2008, 100(22): 1643-1648.
- Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, Harris L, Hait W, Toppmeyer D: Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol 2006, 24(36): 5652-5657.
- Shackleton M, Quintana E, Fearon ER, Morrison SJ: Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell 2009, 138(5): 822-829.
- Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc 2006, 128(6): 2115-2120.
- Patil SJ, Zajac A, Zhukov T, Bhansali S: Ultrasensitive electrochemical detection of cytokeratin-7, using Au nanowires based biosensor. Sensors and Actuators B: Chemical 2008, 129(2): 859-865.
- Nie S, Xing Y, Kim GJ, Simons JW: Nanotechnology applications in cancer. Annu Rev Biomed Eng 2007, 9(1): 257-288.
- Yu WW, Qu L, Guo W, Peng X: Experimental determination of the extinction coefficient of CdTe, CdSe, and CdS nanocrystals. Chem Mater 2003, 15(14): 2854-2860.
- An J, Peng C, Tang H, Liu X, Peng F: New advances in the research of resistance to neoadjuvant chemotherapy in breast cancer. Int J Mol Sci 2021, 22(17): 9644.
- Hu M, Cheng N, Wang S, Li R, Liu Y, Wang L, Chen W, Chen Y: Salvianolic acid B-loaded polydopamine-modified hollow mesoporous organic silica nanoparticles for treatment of breast cancer metastasis via suppressing cancer-associated fibroblasts. Eur J Pharm Sci 2024, 192: 106641.
- Zhang X, Li N, Zhang G, Li J, Liu Y, Wang M, Ren X: Nano Strategies for artemisinin derivatives to enhance reverse efficiency of multidrug resistance in breast cancer. Curr Pharm Des 2023, 29(43): 3458-3466.
- Li J, Zhang W, Gao Y, Tong H, Chen Z, Shi J, Santos HA, Xia B: Near-infrared light and magnetic field dual-responsive porous silicon-based nanocarriers to overcome multidrug resistance in breast cancer cells with enhanced efficiency. J Mater Chem B 2020, 8(3): 546-557.
- Li W, Fu Y, Sun J, Gong H, Yan R, Wang Y: Construction and in vitro evaluation of pH-sensitive nanoparticles to reverse drug resistance of breast cancer stem cells. Discov Oncol 2024, 15(1): 21.
- Guo F, Yu N, Jiao Y, Hong W, Zhou K, Ji X, Yuan H, Wang H, Li A, Wang G et al: Star polyester-based folate acid-targeting nanoparticles for doxorubicin and curcumin co-delivery to combat multidrug-resistant breast cancer. Drug Deliv 2021, 28(1): 1709-1721.
- Wang W, Zhou M, Xu Y, Peng W, Zhang S, Li R, Zhang H, Zhang H, Cheng S, Wang Y et al: Resveratrol-loaded TPGS-resveratrol-solid lipid nanoparticles for multidrug-resistant therapy of breast cancer: in vivo and in vitro study. Front Bioeng Biotechnol 2021, 9: 762489.
- Marra A, Trapani D, Viale G, Criscitiello C, Curigliano G: Practical classification of triple-negative breast cancer: intratumoral heterogeneity, mechanisms of drug resistance, and novel therapies. NPJ Breast Cancer 2020, 6(1): 54.
- Yin J, Lang T, Cun D, Zheng Z, Huang Y, Yin Q, Yu H, Li Y: Erratum: pH-Sensitive Nano-Complexes Overcome Drug Resistance and Inhibit Metastasis of Breast Cancer by Silencing Akt Expression: Erratum. Theranostics 2020, 10(5): 2399-2400.
- Guney Eskiler G, Cecener G, Egeli U, Tunca B: Talazoparib nanoparticles for overcoming multidrug resistance in triple‐negative breast cancer. J Cell Physiol 2020, 235(9): 6230-6245.
- Sarkar R, Biswas S, Ghosh R, Samanta P, Pakhira S, Mondal M, Dutta Gupta Y, Bhandary S, Saha P, Bhowmik A et al: Exosome-sheathed porous silica nanoparticle-mediated co-delivery of 3, 3′-diindolylmethane and doxorubicin attenuates cancer stem cell-driven EMT in triple negative breast cancer. J Nanobiotechnology 2024, 22(1): 285.
- Wang B, Zhang R, Wang Y, Qian H, Wu D, He B, Liao H: Targeting Rab26 to conquer cisplatin-resistant lung cancer with self-assembled DNA nanomaterials. Biomacromolecules 2023, 24(5): 2063-2074.
- Ku TH, Shen WT, Hsieh CT, Chen GS, Shia WC: Specific forms of graphene quantum dots induce apoptosis and cell cycle arrest in breast cancer cells. Int J Mol Sci 2023, 24(4): 4046.
- Zhang L, Ren Z, Lü J, Mo X, Lin J, Li Y, Ma W, Liu P, Shen Y, Zhao Q et al: Nanoparticles carrying paclitaxel and anti-miR-221 for breast cancer therapy triggered by ultrasound. Cell Death Discov 2023, 9(1): 298.
- Li J, Lu W, Yang Y, Xiang R, Ling Y, Yu C, Zhou Y: Hybrid nanomaterials for cancer immunotherapy. Adv Sci 2023, 10(6): 2204932.
- Martín-Pardillos A, Martin-Duque P: Cellular alterations in carbohydrate and lipid metabolism due to interactions with nanomaterials. J Funct Biomater 2023, 14(5): 274.
- Alves AD, Bruinsmann FA, Guterres SS, Pohlmann AR: Organic nanocarriers for bevacizumab delivery: An overview of development, characterization and applications. Molecules 2021, 26(14): 4127.
- Zhang L, Zhu C, Huang R, Ding Y, Ruan C, Shen XC: Mechanisms of reactive oxygen species generated by inorganic nanomaterials for cancer therapeutics. Front Chem 2021, 9: 630969.
- Tran P, Nguyen TN, Lee Y, Tran PN, Park JS: Docetaxel-loaded PLGA nanoparticles to increase pharmacological sensitivity in MDA-MB-231 and MCF-7 breast cancer cells. Korean J Physiol Pharmacol 2021, 25(5): 479-488.
- Boratto FA, Lages EB, Loures CM, Sabino AP, Malachias A, Townsend DM, De Barros AL, Ferreira LA, Leite EA: Alpha-tocopheryl succinate and doxorubicin-loaded liposomes improve drug uptake and tumor accumulation in a murine breast tumor model. Biomed Pharmacother 2023, 165: 115034.
- Badr-Eldin SM, Aldawsari HM, Fahmy UA, Ahmed OA, Alhakamy NA, Al-Hejaili OD, Alhassan AA, Ammari GA, Alhazmi SI, Alawadi RM et al: Optimized apamin-mediated nano-lipidic carrier potentially enhances the cytotoxicity of ellagic acid against human breast cancer cells. Int J Mol Sci 2022, 23(16): 9440.
- Chen T, Chen H, Jiang Y, Yan Q, Zheng S, Wu M: Co-delivery of 5-fluorouracil and paclitaxel in mitochondria-targeted KLA-modified liposomes to improve triple-negative breast cancer treatment. Pharmaceuticals 2022, 15(7): 881.
- Granja A, Nunes C, Sousa CT, Reis S: Folate receptor-mediated delivery of mitoxantrone-loaded solid lipid nanoparticles to breast cancer cells. Biomed Pharmacother 2022, 154: 113525.
- Aly S, El-Kamel AH, Sheta E, El-Habashy SE: Chondroitin/Lactoferrin-dual functionalized pterostilbene-solid lipid nanoparticles as targeted breast cancer therapy. Int J Pharm 2023, 642: 123163.
- da Rocha MC, da Silva PB, Radicchi MA, Andrade BY, de Oliveira JV, Venus T, Merker C, Estrela-Lopis I, Longo JP, Báo SN et al: Docetaxel-loaded solid lipid nanoparticles prevent tumor growth and lung metastasis of 4T1 murine mammary carcinoma cells. J Nanobiotechnology 2020, 18(1): 1-20.
- SS Pindiprolu SK, Krishnamurthy PT, Ghanta VR, Chintamaneni PK: Phenyl boronic acid-modified lipid nanocarriers of niclosamide for targeting triple-negative breast cancer. Nanomedicine 2020, 15(16): 1551-1565.
- Kesharwani P, Chadar R, Shukla R, Jain GK, Aggarwal G, Abourehab MA, Sahebkar A: Recent advances in multifunctional dendrimer-based nanoprobes for breast cancer theranostics. J Biomater Sci Polym Ed 2022, 33(18): 2433-2471.
- Saravanakumar K, Anbazhagan S, Usliyanage JP, Naveen KV, Wijesinghe U, Xiaowen H, Priya VV, Thiripuranathar G, Wang MH: A comprehensive review on immuno-nanomedicine for breast cancer therapy: Technical challenges and troubleshooting measures. Int Immunopharmacol 2022, 103: 108433.
- Jain K, Kesharwani P, Gupta U, Jain NK: Dendrimer toxicity: Let's meet the challenge. Int J Pharm 2010, 394(1-2): 122-142.
- Kim Y, Park EJ, Na DH: Recent progress in dendrimer-based nanomedicine development. Arch Pharm Res 2018, 41(6): 571-582.
- Guo XL, Kang XX, Wang YQ, Zhang XJ, Li CJ, Liu Y, Du LB: Co-delivery of cisplatin and doxorubicin by covalently conjugating with polyamidoamine dendrimer for enhanced synergistic cancer therapy. Acta Biomater 2019, 84: 367-377.
- Yadav P, Ambudkar SV, Rajendra Prasad N: Emerging nanotechnology-based therapeutics to combat multidrug-resistant cancer. J Nanobiotechnol 2022, 20(1): 423.
- Avramovic N, Mandic B, Savic-Radojevic A, Simic T: Polymeric nanocarriers of drug delivery systems in cancer therapy. Pharmaceutics 2020, 12(4): 298.
- Hanafy NA, El-Kemary M, Leporatti S: Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018, 10(7): 238.
- Peng J, Chen J, Xie F, Bao W, Xu H, Wang H, Xu Y, Du Z: Herceptin-conjugated paclitaxel loaded PCL-PEG worm-like nanocrystal micelles for the combinatorial treatment of HER2-positive breast cancer. Biomaterials 2019, 222: 119420.
- Afzal M, Alharbi KS, Alruwaili NK, Al-Abassi FA, Al-Malki AA, Kazmi I, Kumar V, Kamal MA, Nadeem MS, Aslam M et al: Nanomedicine in treatment of breast cancer–A challenge to conventional therapy. Semin Cancer Biol 2021, 69: 279-292.
- Juan A, Cimas FJ, Bravo I, Pandiella A, Ocaña A, Alonso-Moreno C: An overview of antibody conjugated polymeric nanoparticles for breast cancer therapy. Pharmaceutics 2020, 12(9): 802.
- Carreiró F, Oliveira AM, Neves A, Pires B, Nagasamy Venkatesh D, Durazzo A, Lucarini M, Eder P, Silva AM, Santini A et al: Polymeric nanoparticles: Production, characterization, toxicology and ecotoxicology. Molecules 2020, 25 (16): 3731.
- Kafle U, Agrawal S, Dash AK: Injectable nano drug delivery systems for the treatment of breast cancer. Pharmaceutics 2022, 14(12): 2783.
- Miele E, Spinelli GP, Miele E, Tomao F, Tomao S: Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int J Nanomedicine 2009, 4: 99-105.
- Parodi A, Kolesova EP, Voronina MV, Frolova AS, Kostyushev D, Trushina DB, Akasov R, Pallaeva T, Zamyatnin Jr AA: Anticancer nanotherapeutics in clinical trials: The work behind clinical translation of nanomedicine. Int J Mol Sci 2022, 23(21): 13368.
- Cheung A, Bax HJ, Josephs DH, Ilieva KM, Pellizzari G, Opzoomer J, Bloomfield J, Fittall M, Grigoriadis A, Figini M et al: Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7(32): 52553-52574.
- Subhan MA, Yalamarty SS, Filipczak N, Parveen F, Torchilin VP: Recent advances in tumor targeting via EPR effect for cancer treatment. J Pers Med 2021, 11(6): 571.
- Barenholz YC: Doxil®-The first FDA-approved nano-drug: Lessons learned. J Controlled Release 2012, 160(2): 117-134.
- Chen S, Lv Y, Wang Y, Kong D, Xia J, Li J, Zhou Q: Tumor acidic microenvironment-responsive promodulator iron oxide nanoparticles for photothermal-enhanced chemodynamic immunotherapy of cancer. ACS Biomater Sci Eng 2023, 9(2): 773-783.
- Basu SM, Chauhan M, Giri J: pH-Responsive Polypropylene Sulfide Magnetic Nanocarrier-Mediated Chemo-Hyperthermia Kills Breast Cancer Stem Cells by Long-Term Reversal of Multidrug Resistance and Chemotherapy Resensitization. ACS Appl Mater Interfaces 2023, 15(50): 58151-58165.
- Chauhan M, Basu SM, Qasim M, Giri J: Polypropylene sulphide coating on magnetic nanoparticles as a novel platform for excellent biocompatible, stimuli-responsive smart magnetic nanocarriers for cancer therapeutics. Nanoscale 2023, 15(16): 7384-7402.
- Li Y, Wu P, Zhu M, Liang M, Zhang L, Zong Y, Wan M: High‐Performance Delivery of a CRISPR Interference System via Lipid‐Polymer Hybrid Nanoparticles Combined with Ultrasound‐Mediated Microbubble Destruction for Tumor‐Specific Gene Repression. Adv Healthc Mater 2023, 12(10): e2203082.
- Chaudhari R, Patel V, Kumar A: Cutting-edge approaches for targeted drug delivery in breast cancer: beyond conventional therapies. Nanoscale Adv 2024, 6(9): 2270-2286.
- McMullen P, Luozhong S, Tsao C, Xu H, Fang L, Jiang S: A low-immunogenic genetically-fusible zwitterionic polypeptide. Nano Today 2022, 47: 101674.
- Gavas S, Quazi S, Karpiński TM: Nanoparticles for cancer therapy: current progress and challenges. Nanoscale Res Lett 2021, 16(1): 173.
- Abdolvahab MH, Karimi P, Mohajeri N, Abedini M, Zare H: Targeted drug delivery using nanobodies to deliver effective molecules to breast cancer cells: the most attractive application of nanobodies. Cancer Cell Int 2024, 24(1): 67.
- Wang R, Huang X, Chen X, Zhang Y: Nanoparticle-Mediated Immunotherapy in Triple-Negative Breast Cancer. ACS Biomater Sci Eng 2024, 10(6): 3568-3598.
- Marshall SK, Angsantikul P, Pang Z, Nasongkla N, Hussen RS, Thamphiwatana SD: Biomimetic targeted theranostic nanoparticles for breast cancer treatment. Molecules 2022, 27(19): 6473.
- Cheng Z, Li M, Dey R, Chen Y: Nanomaterials for cancer therapy: current progress and perspectives. J Hematology Oncol 2021, 14(1): 85.
- Zhu Y, Wang A, Zhang S, Kim J, Xia J, Zhang F, Wang D, Wang Q, Wang J: Paclitaxel-loaded ginsenoside Rg3 liposomes for drug-resistant cancer therapy by dual targeting of the tumor microenvironment and cancer cells. J Adv Res 2023, 49: 159-73.
- Yang T, Zhai J, Hu D, Yang R, Wang G, Li Y, Liang G: “Targeting design” of nanoparticles in tumor therapy. Pharmaceutics 2022, 14(9): 1919.
- Herea DD, Zară-Dănceanu CM, Lăbușcă L, Minuti AE, Stavilă C, Ababei G, Tibu M, Grigoraș M, Lostun M, Stoian G et al: Enhanced Multimodal Effect of Chemotherapy, Hyperthermia and Magneto-Mechanic Actuation of Silver-Coated Magnetite on Cancer Cells. Coatings 2023, 13(2): 406.
- Tanaka T, Decuzzi P, Cristofanilli M, Sakamoto JH, Tasciotti E, Robertson FM, Ferrari M: Nanotechnology for breast cancer therapy. Biomed Microdevices 2009, 11(1): 49-63.
- Nie S, Xing Y, Kim GJ, Simons JW: Nanotechnology applications in cancer. Annu Rev Biomed Eng 2007, 9(1): 257-288.
- Sinha R, Kim GJ, Nie S, Shin DM: Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther 2006, 5(8): 1909-1917.
- Bleyer AO, O'leary M, Barr R, Ries LA: Cancer epidemiology in older adolescents and young adults 15 to 29 years of age, including SEER incidence and survival: A Children’s Oncology Group and SEER Publication, 1975-2000.
- Massadeh S, Omer ME, Alterawi A, Ali R, Alanazi FH, Almutairi F, Almotairi W, Alobaidi FF, Alhelal K, Almutairi MS et al: Optimized polyethylene glycolylated polymer–lipid hybrid nanoparticles as a potential breast cancer treatment. Pharmaceutics 2020, 12(7): 666.
- I. El-Gogary R, Gaber SA, Nasr M: Polymeric nanocapsular baicalin: chemometric optimization, physicochemical characterization and mechanistic anticancer approaches on breast cancer cell lines. Sci Rep 2019, 9(1): 11064.
- Jithan AV, Madhavi K, Madhavi M, Prabhakar K: Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int J Pharm Investig 2011, 1(2): 119-125.
- Nicolas S, Bolzinger MA, Jordheim LP, Chevalier Y, Fessi H, Almouazen E: Polymeric nanocapsules as drug carriers for sustained anticancer activity of calcitriol in breast cancer cells. Int J Pharm 2018, 550(1-2): 170-179.
- Thadakapally R, Aafreen A, Aukunuru J, Habibuddin M, Jogala S: Preparation and characterization of PEG-albumin-curcumin nanoparticles intended to treat breast cancer. Indian J Pharm Sci 2016, 78(1): 65-72.
- Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W: Co-delivery of rapamycin-and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv 2016, 23(7): 2608-2616.
- Zhang Y, Guo Q, An S, Lu Y, Li J, He X, Liu L, Zhang Y, Sun T, Jiang C et al: ROS-switchable polymeric nanoplatform with stimuli-responsive release for active targeted drug delivery to breast cancer. ACS Appl Mater Interfaces 2017, 9(14): 12227-12240.
- Acharya S, Dilnawaz F, Sahoo SK: Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy. Biomaterials 2009, 30(29): 5737-5750.
- Dehghan Kelishady P, Saadat E, Ravar F, Akbari H, Dorkoosh F: Pluronic F127 polymeric micelles for co-delivery of paclitaxel and lapatinib against metastatic breast cancer: preparation, optimization and in vitro evaluation. Pharm Dev Technol 2015, 20(8): 1009-1017.
- Anzar N, Mirza MA, Anwer K, Khuroo T, Alshetaili AS, Alshahrani SM, Meena J, Hasan N, Talegaonkar S, Panda AK et al: Preparation, evaluation and pharmacokinetic studies of spray dried PLGA polymeric submicron particles of simvastatin for the effective treatment of breast cancer. J Mol Liquids 2018, 249: 609-616.
- Markeb AA, El-Maali NA, Sayed DM, Osama A, Abdel-Malek MA, Zaki AH, Elwanis ME, Driscoll JJ: Synthesis, Structural Characterization, and Preclinical Efficacy of a Novel Paclitaxel‐Loaded Alginate Nanoparticle for Breast Cancer Treatment. Int J Breast Cancer 2016, 2016(1): 7549372.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript