Review Article | Open Access
Insight into the anti-cancer and anti-viral therapeutic properties of biological active molecule prodigiosin
Amna Shafqat1
1Department of Biochemistry, University of Central Lancashire, Preston, Lancashire, PR1 2HE, United Kingdom.
Correspondence: Amna Shafqat (Department of Biochemistry, University of Central Lancashire, Preston, Lancashire, PR1 2HE, United Kingdom; E-mail: Amnashafqat07@gmail.com).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2024, 4: 122-136. https://doi.org/10.32948/ajpt.2024.12.19
Received: 20 Oct 2024 | Accepted: 25 Dec 2024 | Published online: 30 Dec 2024
Key words anticancer, antiviral, immunomodulator, immunosuppressant, prodigiosin
Prodigiosin (PG) is a red-colored pigment belonging to the “prodiginines” group of bacterial secondary metabolites found in gram-negative bacteria Serratia marcescens [12]. Some examples of bacterial prodigiosin are shown in Figure 1. Prodigiosin is a proven anticancer agent and declared most effective against multiple tumor cell lines along with cells that have multi-drug resistance showing a small or negligible effect on normal cell lines [13]. PG not only acts as an anti-cancerous and immunomodulatory agent but also has the potential to kill parasites, insects, and microbes [14]. By viewing all the aspects of Prodigiosin potential against various therapeutic activities there is a keen attention of researchers towards the use of PG as an anticancer agent during the last few decades. It was seen that PG significantly inhibits the mammalian target of the rapamycin (mTOR) mechanism along with angiogenesis. It causes cycle quenching and apoptosis in tumor cell lines with little or no toxicity to normal healthy cells [15]. Innate cytotoxicity is one of the main problems linked with the use of immunosuppressants, especially in oncology, that leads scientists toward the application of combined regimens. In this perception, PG offers an interesting perspective of combinatorial applications. It has a synergistic effect when used in combination with cyclosporin A and acts as an additive when applied with rapamycin, showing its distinctive properties and prospects in the development and use as an immunosuppressant [16, 17].
Though with its widespread anti-cancerous activities, there are no such investigations on its metabolic reprogramming and immunomodulating properties, the information available is only related to a relevant compound known as prodigiosin 25-C [18]. Although PG has therapeutical potential in the discipline of biomedicines there is still no PG-derived medicine available in the market because of some limitations which restrict its entry into the field of medicine. The major and devastating limitation associated with its use is its source, which is an infectious strain: Serratia marcescens, linked with a few harmful pathologies in mammals [19]. The other includes deprivation of genetic evidence, systematic production of prodigiosin in the laboratory and metabolic linkages of other microorganisms that produce prodigiosin. Various studies have been conducted regarding the impact of prodigiosin on breast cancers. Given that proteins play an important role in either activating or suppressing the pathways involved in various types of cancers, it is important to prioritize interlinkage studies between prodigiosin and proteins linked to a particular pathway. These studies are crucial for comprehending the underlying mechanisms behind any type of cancer. The procedures and techniques used in research have significantly improved over time. These developments enable more in-depth analyses of the prodigiosin's characteristics and processes. We may take advantage of these developments to learn more about the effects and prospective uses of prodigiosin by assessing the existing research in this field. In this review, we review methodology, sources of prodigiosin and its biosynthetic pathway. Besides, the immunomodulator role of prodigiosin in different immune cells such as T and B lymphocytes, macrophages, dendritic cells and natural killer cells and its immunosuppressive and antiviral activity will also be covered.
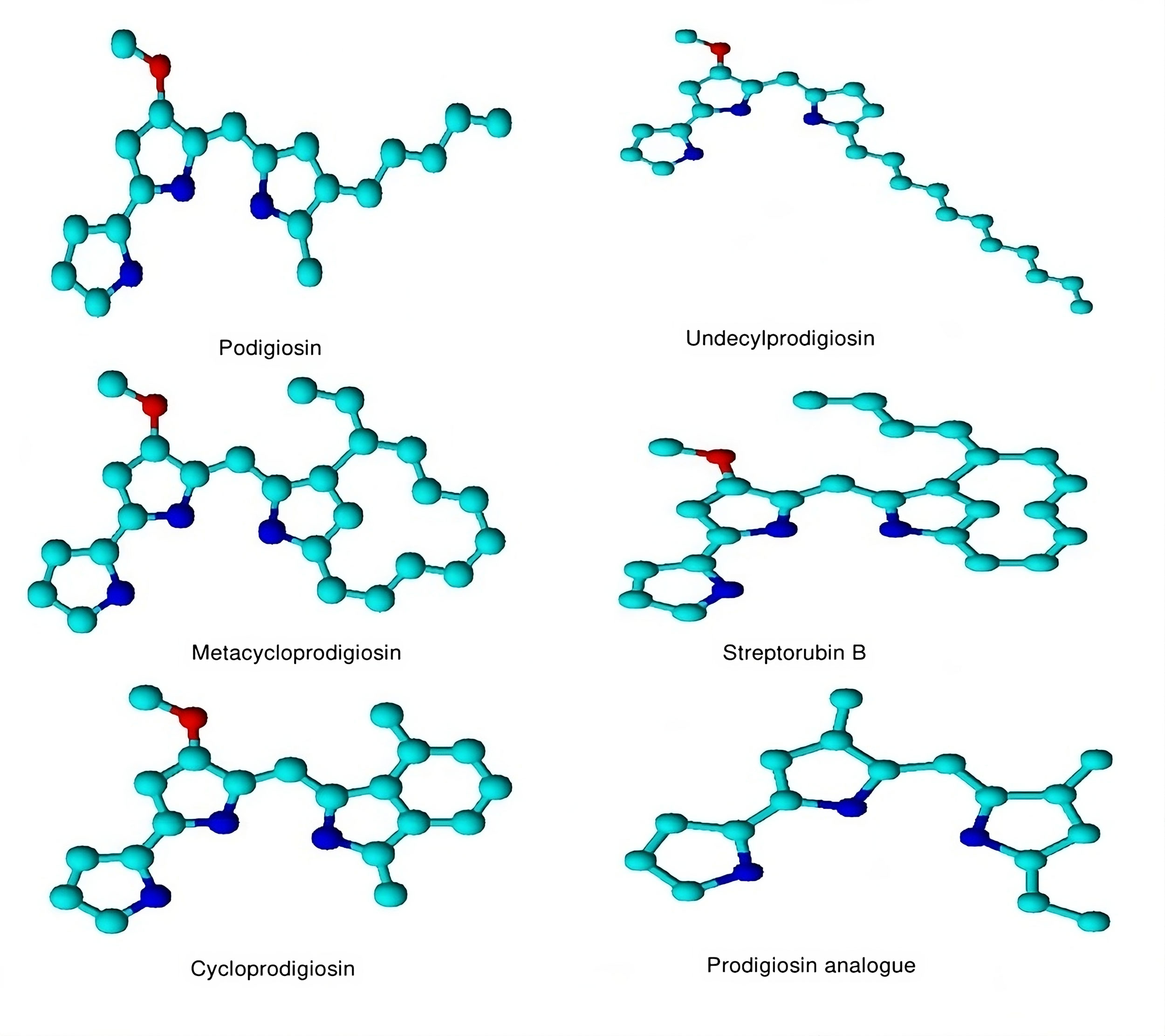 Figure 1. Some compounds of bacterial prodigiosin. Molecular modeling of 3D structures designed by the ChemDraw tool.
Figure 1. Some compounds of bacterial prodigiosin. Molecular modeling of 3D structures designed by the ChemDraw tool.
Generally, carbohydrates were seen to be a poor nutrient source for prodigiosin synthesis because glucose has been described as a suppressor of PG production in S. marcescens [25]. The suppressing impact associated with glucose in the undecyl synthesis of prodigiosin has also been reported in Streptomyces [24]. Along with the C source, the kind of N2 source in the media and the carbon-to-nitrogen ratio also affect PG synthesis. Su and co-workers had recent reports and specified a precise and accurate concentration of inorganic phosphates and peptone for the synthesis of PG by S. marcescens up to the levels of 2.4 g l-1 using response surface methodology. They also evaluated the significance of glycine and sucrose for even higher production yields [26]. In the same way, Chen and coworkers acquired an increased PG production of approximately 15.6 g l-1 by S. marcescens C3 performing a statistically designed experiment. They defined carbon to nitrogen proportion as 6: 4 (starch: peptone) and use immobilization methodology [27]. Up till now, the best source of PG production, which is S. marcescens, has not been extensively implemented for its commercial usage. This might be due to the disadvantage linked with this cunning living being which is a harmful human pathogen and associated with many outbreaks and nosocomial diseases [28]. However, the other traditional industrially proven strains such as Streptomyces may be found to be a better candidate for the high-level synthesis and optimization of prodigiosin (Figure 2).
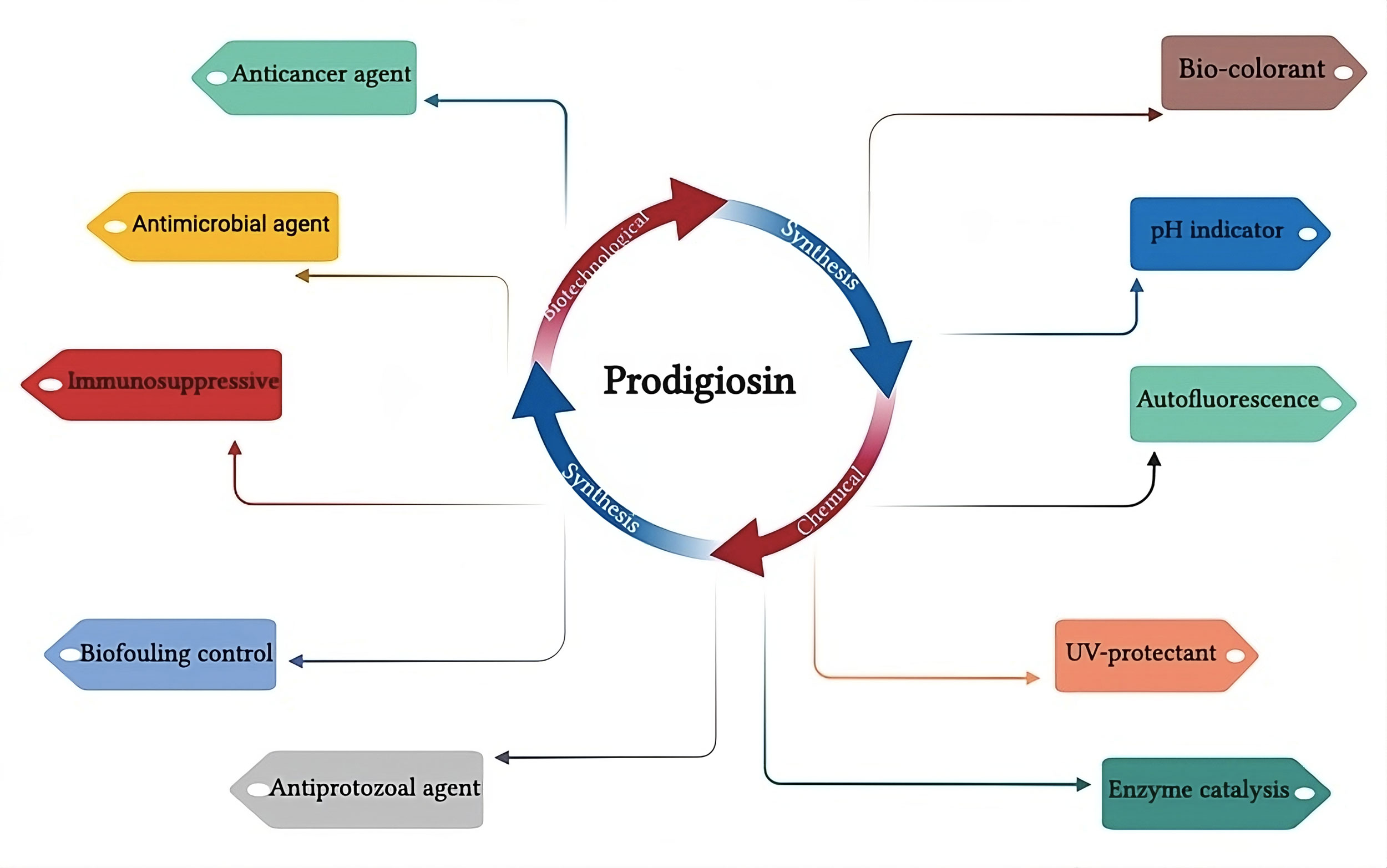 Figure 2. Prodigiosin synthesis pathways and its different biological applications.
Figure 2. Prodigiosin synthesis pathways and its different biological applications.
It has previously been seen that there are 12 out of 14 genes in pig clusters are designated and characterized through the cross-feeding-based study of individual gene mutants [33]. Genes that were allocated to produce the mono pyrrole moiety, MAP were pigB, pigD and pigE, whereas the genes accountable for the production of bipyrrole moiety MBC, were pigA, pigF, pigG, pigH, pigI, pigJ, pigM and pigN. The gene pigC performs the function of encoding the enzyme for terminal condensing of both MAP and MBC for the final synthesis of prodigiosin. There are sequence similarities between the N and C terminal domains of pigC gene with that of phosphoryl transferase moiety and the binding sites of ATP of pyruvate phosphate dikinase enzymes, respectively [29]. According to BLASTP analysis, it was seen that pyruvate phosphate dikinase PEP/pyruvate binding domain is present in both PigC and pyruvate phosphate dikinase of bacteria S. marcescens with 99% similarity. This enzyme is specialized in catalyzing the binding of MBC and MAP together with a core catalytic site for the synthesis of PG [32]. The task assigned for pigK and pigL genes is presently unidentified, but it was assumed that pigK might be involved in the folding of other Pig enzymes and act as a molecular chaperone in the biosynthesis of MBC. Moreover, PigL, which is known to be a 4′- phosphopantetheinyl transferase, could take part in the phosphopantetheinylation reaction in the MBC mechanism [33].
Anti-cancer activity
A number of studies have been performed to evaluate the role of PG in cancer treatment and prevention (Table 2). Proapoptotic anti-cancer activity of prodigiosin has been observed and described to provoke cellular stresses such as DNA impairment, cell cycle blockage and fluctuations in intracellular pH, all of which can influence apoptosis (Figure 3) [39]. Initial studies had reported that PG stimulates apoptosis in tumor cell line independent of its mechanism of action. The phenomenon of apoptosis induction has been seen in various individual tumor cells, in hepatocellular carcinoma xenografts, in tissue culture and human primary tumor cells [40]. Mitochondrial dysfunction and ATP depletion is widely used and acknowledged anticancer pathway [41]. Francisco et al. (2007) reported that PG act as a hydrogen acceptor which could eradicate intracellular pH concentration leading towards disconnection of transport in the electronic chain of proton to ATP synthase of mitochondria, therefore initializing ATP reduction followed by apoptotic cytotoxicity in neuroblastoma cell lines [42]. Llagostera et al., (2003) also described the mitochondrial mechanism-dependent cytochrome C release which leads toward PG-induced apoptosis in lung tumor cell lines [43].
Interestingly, PG was seen when it was treated with breast tumor cell lines; estrogen receptor-positive (MCF-7) and negative (MDA-MB-231) and the MCF-7 cells which show multidrug-resistant (MDR). In this study, it was observed that PG acts as an apoptotic inducer following the mitochondrial mechanism producing cytotoxicity on the three cell lines in a time and dose-dependent manner. Although PG has negligible or no effect on MCF-7 tumor cell lines, therefore it is diminishing the reality that PG might act as a substrate for MDR transporter fragments [40]. In the era of mitochondrial pathway-dependent apoptosis, there are some studies suggesting another mechanism comprising double-stranded DNA cleavage that leads to apoptosis in cancer cell lines. It has also been observed that this red pigment stimulated H+/Cl− symporter protein by uncoupling of vacuolar H+ ATPase as it has the potential of electrostatically binding with negatively charged Cl molecule inducing proton paired transport of halides in transmembrane. This phenomenon of binding suggests that PG has proven to be a promising pH activator to target tumor cell lines with enhanced intracellular pH gradients [44]. The apoptotic induction of prodigiosin was examined on the gastric cancerous cell of humans (HGT-l) resulting in a continued deprivation in cell feasibility initiated by apoptotic cell death. Other morphological changes occur in carcinoma cell lines when treated with PG, for example, shrinking of cells, condensing of chromatin, etc [45].
In vitro and in vivo studies of treating PG against lung cancer cells including doxorubicin-resistant (Dox-R) and doxorubicin-sensitive (Dox-S). Similar kind of results was obtained for PG cytotoxic potential against these cells and a maximal half concentration10 μM. The phenomenon of apoptosis was categorized as autophagy, but also apoptotic features were observed by a subpopulation of cells. Moreover, tumors present in the mice trachea were mitigated showing a clear induction of cytotoxicity with PG treatment in lung cancer cells of both Dox-R and Dox-S [46]. PG-induced cell deaths in breast tumor cell lines were seen using the cell culture technique as well as in vivo methodology. The results indicated that PG has the potential of blocking Wnt/β-catenin signaling, which has a significant role in breast cancer propagation and development and hence controls cancer cell growth, invasion, and migration [47].
PG was treated against both ovarian cell lines (over-expressing BCRP, MDR1, or MRP2 pumps) their non-MDR type cell lines and multidrug-resistant human gastric cell line. It was observed that this red pigment induced a similar cytotoxic impact to the parental cell lines with negligible difference in comparison with mitoxantrone, daunorubicin and cisplatin. In addition to this FACS analysis described that prodigiosin cannot be exported out of human epithelial ovarian and gastric cancer cells unlike that of mitoxantrone or daunorubicin which can be efficiently transferred by the ABC pumps [48]. Those findings were indicating that prodigiosin was not performing the function of substrate for MDR receptor protein and due to this reason, it could be a significant tool to treat tumor cell lines that overexpress MDR transporters.
Another study suggested potent anticancer activities of pure prodigiosin which is isolated from the marine chitin’s fermentation against HepG2, A549, WiDr and MCF-7 cell lines. The IC50 values were compared with the well-known allopathic anti-cancer drug Mitomycin C which is 2.75-fold, 1.67-fold and 3.25- folds more efficient as compared to this drug [49]. A study was conducted to treat purified PG on human prostate tumor cell lines (PC3) and choriocarcinoma (JEG3). In an in vitro analysis prodigiosin induced apoptosis in JEG3 cell lines in a dose-dependent manner. Moreover, an interesting phenomenon of dose and time-dependent JEG3 and PC3 tumor cell growth inhibition was seen via vivo assay. It was assumed that this pigment activated a cell death response majorly due to the mitochondria-cytochrome c release mechanism with the production of caspase- and caspase-9 stimulation following the poly (ADP-ribose) polymerase (PARP) proteolysis [50]. Another study reported a similar kind of mechanism of action related to prodigiosin treatment against GLC4, its derivative doxorubicin-resistant GLC4/ADR and two small cell lung carcinoma (SCLC) cell lines. A dose-dependent apoptotic effect was seen produced by cytochrome C release, caspase cascade trigger and breakage of PARP. In addition to this, the study described that the red pigment can reduce the phenotype which showed multidrug resistance because it has no variations among the two cell lines [51].
The combined effect of prodigiosin was studied with purine analog PU-H71, which is perfectly soluble in water and is considered to have a greater affinity towards tumor cell lines and is in phase I clinical trial testing. The study was conducted against triple-negative breast cancer MDA-MB-231 cells in a dose-dependent manner. The IC50 values for prodigiosin (2.1 μM) and PU-H71 (157.88 nM) were determined based on the percentage of inhibition, indicating that they were capable of killing 50% of the MDA-MB-231 cells. Moreover, when combining half of the IC50 values for both drugs, the highest percentage of inhibition (75.14%) was observed in comparison to other patterns. The combined impact of both components also causes morphological changes in terms of a higher quantity of floating cells, with a more spherical shape of the cell. These morphological changes were associated with greater cytotoxicity in comparison with untreated cells and cells treated with DMSO, indicating a collaborative effect that was described by the CI method (CI = 0.7). This collaborative effect was seen among both modules due to the increased level of caspase 3, 8, and 9 which is relevant to apoptosis induced by PG. Additionally, a significant reduction in the levels of Heat shock protein 90 (HSP90α) was seen which is a small chaperone family and is regarded as the crucial activator for the transcription of proteostasis and expression levels related to PU-H71 action [52].
PG can inhibit cell proliferation of cholangiocarcinoma (CCA) by suppressing the phenomenon of autophagy which is SNAREs-dependent, showing that it might be a promising drug to be used as a chemotherapeutic agent for advanced CCA [53]. Treatment of PG with a concentration of 30-100 mg/mL for 4–7 hours against THP-1 cells decreased cell viability. Tumor cells after prodigiosin treatment when compared with non-tumor epithelial Vero normal kidney cell lines, showed that normal cell lines are not susceptible to PG doses [54]. PG showed in vivo anti-cancerous activity in BALB/c mice against Lewis lung carcinoma-induced tumors. After 28 days of treatment with PG tumor volume decreased by 34.18% [55].
T lymphocytes
Regardless of their use as an antigen-directed antitumor cytotoxic molecule, prodigiosin also triggers T cells over activation (i.e., T cell exhaustion) that induces T cell senescence with deficiencies in effector role and propagation which either avoid or control tumor [56, 57]. The continuous exposure to antigens, assists cancer escape immune surveillance and causes dysfunction of T cells leading towards multiple inhibitory receptors on dysfunctional T cells such as PD-1 [58]. It was investigated through in vitro and in vivo protocols that prodigiosin only targets and inhibits the propagation and T cells immune functioning with no effect on B-cells [59]. However, there is still insufficient information available to say confidently whether prodigiosin impedes the functioning of T cells directly or indirectly. In an analysis, it was observed that PG also prevents graft versus host disease (GvHD) by depriving expression of IL-2Rα in the IL-2/IL-2R signaling pathway to stop T-cell stimulation. It also suspended the proliferation of autoimmune diabetes mellitus causing little or no toxicity to mice [60]. PG 25-C (a relevant compound) can directly attack the stimulated CD8+ T cells by limiting the pH increase associated with intracellular organelles which are mainly needed by cytotoxic T lymphocytes (CTLs) for proper functionality [61]. PG is known to be an efficient compound in an immunosuppressive TME mainly characterized by T cell dysfunction. Moreover, it could also be a significant moiety for T cells related immunological analyses. The research on the level of T-cell obstruction, when treated with PG is important because inhibition of defective T cell is the main root of autoimmune diseases, whereas tumor mainly arises due to inhibition of T-cells to a greater extent [62].
B lymphocytes
There is little information on the effects of prodigiosin on B cells irrespective of its existing consent regarding its immunosuppressive function on T-cells [63]. In breast cancers, B cells constitute 40% of tumor-infiltrating lymphocytes (TILs) and they comprise 25% of all cells in other cancers [64]. B cells have both effects on tumor growth and inhibition, for example, they obstruct tumors by stimulating T cell responses and regulate tumor growth by supporting immune complex formation or immunosuppression via complement activation [65]. Prodigiosin repressed polyclonal B cell propagation, Epstein Barr virus (EBV) and human peripheral blood lymphocytes (PBLs) immortalization. The distinctive prodigiosin response on T and B cells might be endorsed to the source of the cells used for experimental analysis. For instance, human cells reported selective inhibition of T-cell propagation in comparison to mice cells [66]. In addition to this B cells are miscellaneous and assorted which have the potential to increase T cell antitumor activity and it might assist carcinogenesis through angiogenesis, inflammation, and immunosuppression [64].
Macrophages
Current knowledge supports the major function of PG in moderating tumor-associated macrophages (TAMs). Drafted macrophages linked with the Tumor microenvironment (TME) are transformed into TAMs some kind of immunosuppressive macrophages which encourages “tumor tolerance” by destroying the production and functionality of antitumor T cells [67]. Solid tumors, for instance, breast and prostate cancer had storage of TAMs which deal with insignificant disease prediction [68]. PG acts as a pro-apoptotic drug and has significant therapeutic potential against both vascular endothelial growth factor (VEGF) and epidermal growth factor receptor (EGFR). It could also control cancer proliferation by impeding the production of tumor-associated macrophage penetration and M2 polarization [69]. Involvement of phosphatidylinositol 3-kinase (PI3K/Akt) mechanism in the stimulation of tumor-associated macrophages and the PG inhibitory effect on this pathway, presented that this red pigment might control recruitment of TAM and stimulate apoptosis related to tumor necrosis factor (TNF) [70]. Metastasis could also be mediated by TAMs-produced matrix metalloproteinase-9 (MMP-9) and VEGF in primary lung cancer tissues of the TNBC mouse model [71]. Prodigiosin control matrix metalloproteinase-9 (MMP-9) which secretes ß VEGF responsible for the stimulation of cancer growth and angiogenesis associated with TAM [72].
Prodigiosin might also have the potential to disrupt the functionality of nicotinamide adenine dinucleotide phosphate oxidase (NOX) released by TAMs or deprive its activation in the TME, controlling carcinogenesis related to oxidative stress. TAMs release cytokines and chemokines (e.g., TNF-α, prostaglandin E2 [PGE2] and interleukins [ILs],) that explicit NOX2 and assist carcinogenesis, that sustains metastasis, tumor genesis, and immunological tolerance [73]. Prodigiosin Equivalent block stimulation of NOX by interfering with the transfer of Rac protein and p47phox to the cell membrane of mouse macrophage cell line [74]. Prodigiosin decreases metastasis by targeting NOX2 to permit future research, taking into account various factors such as a source of reactive oxygen species (ROS), susceptibility to ROS toxicity, sensitivity to ROS‐induced immunosuppression, tumor cells, effector cells and cancer propagation stage. Macrophage M1 stimulation through IFN-γ is significantly important in immune functionality and plays a role in damaging tissue culture by proinflammatory cytokines [75]. For instance, the transformation of TAMs into immunostimulatory cells is controlled by IFN-γ, suggesting the antitumor immunotherapy's efficiency by producing T cells effector in ovarian tumors [66].
Dendritic cells
Prodigiosin might also moderate TADC's immune functions via PGE2 in the same way as TAMs. Despite that DCs activate an antitumor T-cell immune feedback, malignant tumors acquire other kinds of DCs with lowered relocation and storage in lymphoid organs leading to immunosuppressant T cells. Higher levels of PGE2 switched the immunostimulatory DCs into immunosuppressant T cells to decrease the progression of antitumor T cells by stimulating PD-L1 [76]. Prostaglandin E2 (PGE2) blocks MHC II illustration and activates IL-10 via EP2 and EP4 receptors, diminishing dendritic cells antigen performance initiated through the COX-2/EP3 signaling [77]. The prodigiosin immune-regulatory mechanism of actions on tumor-associated macrophages described previously showed that it can distress dendritic cells in the Tumor microenvironment. PG could alternate TAM-initiated mitigation of tumor antigen-presenting behaviors and tumoricidal due to the established metabolic crosstalk occurring in dendritic cells [78].
Natural killer cells
Regulated natural killer cells eradicate cancer through death receptor-stimulated killing, cytokine generation (i.e., IFN-γ) and granule exocytosis, which mediate other immune cells [79]. Nevertheless, PGE2 per se trades off NK cell activities (e.g., tumor lysis) for metastases development via activated EP2 and EP4 receptors [80]. PGE2 controls the NK cell's functionality through various processes, for example by restraining IFN-γ assembly and ILs–produced IFN-γ expression in natural killer cells through EP2 receptors or reducing natural killer receptors via cAMP/PKA mechanism [76]. EP4 antagonist prostaglandins E2-stimulated natural killer cell destruction by shielding IFN-γ induction by natural killer cells, suppressing lung and breast cancer metastases [81]. PG could have an immunoregulatory function since the reciprocal natural killer-dendritic cells crosstalk is obstructed by prostaglandins E2 via cytokine and chemokine modulation [82].
Immunosuppressive activity
As an immunosuppressant, the potential of members from the prodiginine family to block the cell cycle has been oppressed, at non-apoptotic doses. PG was observed to lower graft versus host disease (GvHD) with no significant symptoms of toxicity in mice modules. Prodigiosin also delayed the progression of autoimmune diabetes mellitus. There was also inhibition of graft versus host disease and collagen-produced arthritis by prodigiosin in mice modules [60]. Different types of PGs like metacycloprodigiosin, Undecylprodigiosin and cyclo-prodigiosin, all have the potential to selectively prevent T cell propagation. However, in vitro, cytotoxicity with different levels was seen. PGs have been presented to inhibit the cell cycle at various phases. The diverse biological activities have been ascribed to their distinct assemblies and/or mechanism of action [83].
Prodigiosin prevents Jurkat T cell proliferation in humans during the G1/S phase. The function of PG in the late G1 stage was represented by reduced expression of cdk2, cyclin E, and cdk4, all of which are shown to be expressed during the mid to late G1 stage of the cell cycle. Moreover, PGs have the potential to lower retinoblastoma Rb protein phosphorylation, which is considered an important initiator for the development of the S phase from the G phase [63]. In leukemic Jurkat cells, inhibition of cyclin E, cdk2, p27, p21, and Rb phosphorylation was also seen followed by apoptosis [83]. Prodigiosin has also the capability to interfere with the accumulation of p53 and the production of NAG - 1, in the breast tumor cells (Mcf – 7) in humans [84]. Prodiginines do not interfere with IL-2 expressions, which makes a complicated structure with its receptor (IL-2R), leading towards another significant turning point in T cell propagation. In addition, numerous kinds of activities relayed to given PGs have been shown at this stage in the cell cycle.
Anti-viral activity
The antiviral potential of PG has little investigation. However, it was seen that PG has the potential to inhibit signal transduction processes which are known to be important for viral infection, due to which there is an increasing trend in today’s research toward the evaluation of PG as an antiviral drug [85]. An in-silico experiment was performed via homology modeling and molecular docking analysis against various viruses to evaluate the antiviral effect of PG. The study analyzed the potential of PG against diverse viral proteins from different viruses such as hepatitis C virus (HCV), hepatitis B virus (HBV), influenza A virus (H1N1) and human immunodeficiency virus (HIV). The study reported that prodigiosin has significant antiviral potential against the hepatitis B virus, human immune deficiency virus, and H1N1 but not for the hepatitis C virus. Even though, these results needed further investigations and research through in vivo and in vitro analysis [86].
The earliest analysis which demonstrated the antiviral effect of prodigiosin was described by Zhou and his coworkers [87]. The researchers investigated the capability of prodigiosin to reduce infection in silkworm BmN cells caused by Bombyx mori nucleopolyhedrovirus (BmNPV). They tested in vitro cell models for human DNA virus predicted to be effective for finding the antiviral drug. The findings showed that prodigiosin at a concentration that is non-cytotoxic to normal cells (300 nM) is shown to have a strong toxic effect on BmN cells. Certainly, PG with concentration (300 nM) deprived the formation of occlusion bodies (OB) that showed the ultimate lytic phase of BmNPV, and production of budded virus. An experiment performed indicated that prodigiosin showed both cytotoxic and antiviral potential against BmNPV and BmNPV infected cells, respectively in time and dose-dependent manner. The antiviral effect of PG in a concentration (10-300 nM) dependent manner was employed for analysis, the pathway followed by PG for antiviral activity comprised of transcription of genes i-e; early (ie-1) gene, which involved in DNA replication, late (vp39) gene, that takes part in capsid formation and very late (p10) gene which plays role in OB production followed by DNA replication of the virus. After exposure of these three genes ie-1, vp39, and p10 to 100 nM prodigiosin for 72 hours, gene expression was repressed 55 to 100-times, indicating that PG can inhibit gene transcription in viruses and hence decrease both OB and BVgeneration.
Interesting research was conducted to evaluate the antiviral effect of prodigiosin against Herpes simplex virus (HSV) 1 and 2 reported by Suryawanshi et al. [17]. The results described that human corneal epithelial (HCE) cells infected by HSV-1 infection are strongly inhibited by 2.5 μM PG, which is a physiologically significant in vitro model. PG with a concentration as low as 0.3 μM inhibited protein synthesis, gene transcription, virus replication and egress of infective virus molecules. In vivo analysis in a mice module, BALB/c suggested that PG also has the potential to lower corneal HSV-1 infection. The mice were poisoned with HSV-1infection and topically treated with prodigiosin, a standard drug Trifluoro thymidine (TFT) used to treat ophthalmic HSV infection and dimethyl sulfoxide as blank with a concentration of 50 μM of both drugs, employed after two days for continuous seven days. Prodigiosin showed significant protection in mice against the development of disease at a level comparable to standard drugs. Additionally, the prophylactic potential of prodigiosin against HSV disease was assayed in HCE cell lines. The researchers found that prodigiosin produced a prophylactic effect, but it had no concern with virus entry, suggesting that prodigiosin only affects the signaling mechanism of the host after the cells got infected by the virus. More, precisely, they evaluated that prodigiosin induced its antiviral effect via inhibiting apoptosis and suppression of NF-κB and Akt signaling which are responsible for host cell existence and is stimulated during infection caused by HSV.
Another study performed on the prodigiosin-derived compound known as obatoclax was reported to have an antiviral effect against alphaviruses like Semliki Forest (SFV) and Chikungunya (CHIKV) viruses [88]. The action pathway of obatoclax was proposed to inhibit the viral combination with cells stimulated by endosomal pH neutralization. Moreover, obatoclax antiviral activity on SARS-Cov-2 indicated the inhibition of replication in epithelial cell cultures. It was seen that the pathway for inhibition was also ascribed mainly due to a decrease in endosomal pH and diminishing activities of cathepsin and furin; enzymes responsible for viral fusion protein stimulation [89, 90].
A dose-dependent in vitro and ex vivo PG analysis against the HSV-1 virus described that PG inhibits replication of the virus. In vivo activity on HSV-1 ocular infection found that PG plays a protective role against disease development. Histopathological studies described that PG preserved the corneal integrity and no inflammation of epithelial cells. The mechanistic study demonstrated that PG inhibits pro-viral host factors including NFkB/protein kinase B (AKT). These findings represent the evidence that during infection, prodigiosin worked out by inhibiting dysregulation of multiple signaling mechanisms, it also interferes with the host system to lower replication of the virus and proliferation [91].
The potato virus Y (PVY) is a plant virus that causes remarkable crop losses globally, particularly in Solanaceae crops. Researchers have determined a strain of the plant growth-promoting rhizobacterium (PGPR), Serratia marcescens-S3, which has the potential to control PVY replication in Nicotiana benthamiana. Although, there is a lack of inclusive studies demonstrating the underlying mechanism. In a recent experiment designed by Ge M et al. (2022), it was observed that the ubiquitination of NbHsc70-2 plays a pivotal role in provoking induced systemic resistance (ISR) by Serratia marcescens-S3. Following treatment with S. marcescens-S3, the protein level of NbHsc70-2 was significantly reduced. Inhibition of ubiquitination led to the increased aggregation of NbHsc70-2 in plants and reduced the S. marcescens-S3-mediated resistance to PVY. Moreover, transgenic Nicotiana benthamiana plants, NbHsc70-2KO and NbHsc70-2USM, were developed using CRISPR-Cas9 technology to knock out and ubiquitinate NbHsc70-2, respectively. S. marcescens-S3 was found to have a remarkable impact on reducing the inhibition of NbHsc70-2 protein aggregation in NbHsc70-2KO and NbHsc70-2USM plants. Importantly, the virulence of PVY was more noticeable in NbHsc70-2USM plants compared to the plants with the wild-type genotype. These findings express that S. marcescens-S3 enhances the ubiquitination process of NbHsc70-2, leading to the suppression of NbHsc70-2 molecular chaperone recruitment and hence reducing the replication as well as infection of PVY [121].
In a recent experiment conducted by Song K et al. (2023), the researchers investigated the Micropterus salmoides rhabdovirus (MSRV), the most lethal viral pathogen in largemouth bass farming, which poses a threat to the survival and health of bass fry. The experiment focused on observing a red-pigmented bacterial strain, Serratia marcescens MS01, present in the gut of M. salmoides. However, the researchers discovered that methanol extracts of sediment (SED) from S. marcescens MS01 showed extremely efficient anti-MSRV potential. At a concentration of 30 mg/L, SED effectively lowered the virulence of MSRV, with the inhibition rate of glycoprotein and nucleoprotein expression reaching 99% in GCO cells. Furthermore, SED inhibited MSRV-induced oxidative stress and enhanced the potential of antioxidant enzymes in CAT and SOD. Subsequent purification, activity analysis, and multi-spectroscopic identification suggested that prodigiosin played a significant role in inhibiting MSRV in SED. These outcomes recommend that SED or prodigiosin have the potential to be employed as promising antiviral approaches against MSRV in aquaculture farms [122].
|
Table 1. In vitro anticancer activity of bacterial prodigiosin against human cell lines. |
|||
|
Compound |
Cell line |
Feedback |
Ref |
|
Prodigiosin |
Human colon cancer/HT29, DLD-1 |
Induces apoptosis in colon cancer |
[92] |
|
Human neuroblastoma/LAN-1, IMR-2, SH-SYSY |
Enhance antitumor agents in the treatment of neuroblastoma |
[42] |
|
|
Human breast carcinoma/MCF-7, MDA-MB-231 |
Apoptosis induced by PG in the MCF-7-MR cell line generates stable fragments of human type-I cytokeratins |
[93] |
|
|
Human lung carcinoma/A549, NCI-H460 |
PG induces apoptosis in both caspase-dependent and caspase-independent pathways |
[43] |
|
|
Human leukemia/U937, Jurkat-T, NSO |
Caspases were activated in apoptotic cells |
[94] |
|
|
Undecylprodigiosin |
Human lung carcinoma/A549 |
Induces significant cytotoxic activities against A-549 |
[95] |
|
Human breast carcinoma/BT-20, MCF-7, MDA-MB-231 |
Induces p53-independent apoptosis |
[96] |
|
|
Metacycloprodigiosin |
Human lung carcinoma/A549, SPCA4 |
Induces significant cytotoxic activities against A-549 and SPCA4 |
[96] |
|
Cycloprodigiosin |
Human breast carcinoma/MDA-MB-231 |
cPrG · HCl treatment suppressed the growth of human breast cancer cell lines by inducing apoptosis |
[97] |
|
Human leukemia/HL-60 |
cPrG · HCl through apoptosis and differentiation induction may be useful in leukemia treatment |
[98] |
|
|
Table 2. Antitumor mechanisms of prodigiosin. |
||||
|
Organ |
Cell Line |
Mechanism |
IC50 |
Ref |
|
Lung |
95-D
|
Inhibiting migration, downregulating RhoA gene expression and protein levels, upregulating cell aggregation |
5.0 μM |
[99] |
|
A549
|
PI3K-p85/Akt/mTOR; PKB/SKP2/p27, upregulating p27KIP1 expression |
0.42 μM |
[46] |
|
|
CL1-5
|
PKB/SKP2/p27, stabilizes p27KIP1 through transcriptional repression |
0.42 μM |
[100] |
|
|
GLC4
|
Intrinsic apoptosis, upregulating cytochrome c release and activating caspase cascade |
- |
[45] |
|
|
GLC4/ADR
|
Intrinsic apoptosis, upregulating PARP cleavage |
- |
[101] |
|
|
H23
|
PKB/SKP2/p27 |
0.42 μM |
[100] |
|
|
Colorectal |
DLD1
|
c-Jun/DNp73/p73/apoptosis; Lysosomal acidification, upregulating apoptosis |
>1.6 μM |
[102] |
|
HCT116
|
c-Jun/DNp73 p73 activation, accumulation of LC-3B- II and SQSTM |
4.0 μM |
[103] |
|
|
HT29
|
p21/cell cycle blockage/ intrinsic apoptosis, upregulating caspase 3 and P53 protein levels |
- |
[92] |
|
|
SW480
|
c-Jun/DNp73 p73 activation, downregulating lysosomal activity by accumulating EGFP-LC3 puncta |
- |
[104] |
|
|
SW-620
|
Apoptosis, upregulating caspase level |
0.273 μM |
[105] |
|
|
Blood |
B-CLL
|
Apoptosis, downregulating vacuolar ATPase |
0.116 μM |
[106] |
|
CCRF-CEM
|
MMP-9, upregulating caspase-3 and apoptosis, downregulating proliferation rate and viable cell number |
- |
[107] |
|
|
HL-60
|
genomic damage/apoptosis PKC/PTP1B/PP2A |
9.7 μM |
[83] |
|
|
Jurkat
|
TopoI inhibition/ p27/p21/cdk2/cyclin-E/apoptosis PKC/p38/MAPK, downregulating number of viable cells
|
4.48 μM |
[108] |
|
|
K562
|
PKC/PTP1B/PP2A, increases caspase 3, caspase 8, caspase 9 |
2.5 μM |
[109] |
|
|
NSO
|
Apoptosis, acts in the absence of P53 |
15–30 μM |
[42] |
|
|
Ramos
|
No significant toxicity, apoptosis and decrease in normal cells |
9.36 μM |
[110] |
|
|
T-ALL
|
MMP-9, upregulation of caspase-3 and accumulation of P53, downregulation of survivin protein levels and number of viable cells |
- |
[110] |
|
|
U937
|
PKC/PTP1B/PP2A |
0.7 μM |
[84] |
|
|
Brain |
GBM8401
|
ER stress/autophagy, reduce cell viability |
- |
[111] |
|
IMR-32
|
ATP production |
0.7 ± 0.1 μM |
[112] |
|
|
LAN-1
|
ATP production |
1.5 ± 0.1 μM |
[113] |
|
|
SH-SY5Y
|
ATP production |
1.5 ± 0.2 μM |
[113] |
|
|
SK-N-AS
|
ATP production |
7.0 ± 0.5 μM |
[113] |
|
|
U87MG
|
ER stress/autophagy, reduce cell viability |
- |
[113] |
|
|
Breast |
MCF-7
|
Genomic damage/ER stress; Akt/GSK-3b/NAG-1, upregulating caspase 3, caspase 7 levels, reduces surviving transcriptional levels |
4.0 μM |
[112] |
|
MCF-7 MR
|
Type I cytokeratin |
- |
[114] |
|
|
MDA-MB-231
|
ER stress; Wnt/b-catenin; JNK/MAPK/RAD51, upregulating caspase 3, caspase 7, caspase 8 levels, reduces RAD51 mRNA expression |
- |
[115] |
|
|
MDA-MB-468
|
Inhibit Wnt/b-catenin, induces apoptosis, reduces cell viability, proliferation |
- |
[116] |
|
|
T47D
|
Cell cycle blockage/ER stress; JNK/MAPK/RAD51, increase caspase 3 and Bax expression levels |
- |
[47] |
|
|
Oral |
OECM1
|
Akt/mTOR/beclin-1/autophagy, arresting cell cycle in G0/ G1 phase |
- |
[92] |
|
SAS
|
Akt/mTOR/beclin-1/autophagy, arresting cell cycle in G0/ G1 phase |
- |
[117] |
|
|
Skin |
SK-MEL-28
|
mTORC1/2 inhibition, cell cycle arrest at G0/ G1 phase, increase apoptosis and DNA damage |
4.51 μM |
[117] |
|
SK-MEL-5
|
mTORC1/2 inhibition, activates the mitochondrial apoptotic pathway, disrupts MCL-1/BAK complexes |
1.02 μM |
[118] |
|
|
Liver |
HepG2
|
Survivin, changes cellular morphology to apoptotic types |
10-39 μg/mL |
[118] |
|
Nasopharyngeal |
CNE2
|
Cell cycle arrest, blocked autophagy |
- |
[119] |
|
Prostate |
PC3
|
Intrinsic apoptosis, downregulate cell and tumor growth |
- |
[120] |
|
Trophoblast |
JEG3
|
Intrinsic apoptosis, upregulate caspase 3, caspase 9 levels and P53 expression levels |
>10 μg/mL |
[50] |
|
Uterus |
Hela
|
Intrinsic apoptosis, antiproliferative effects |
0.5–2.1 mg/mL |
[50] |
|
Stomach |
HGT-1
|
Microtubule alteration, increase apoptosis and decrease cell viability |
3.1 μM |
[101] |
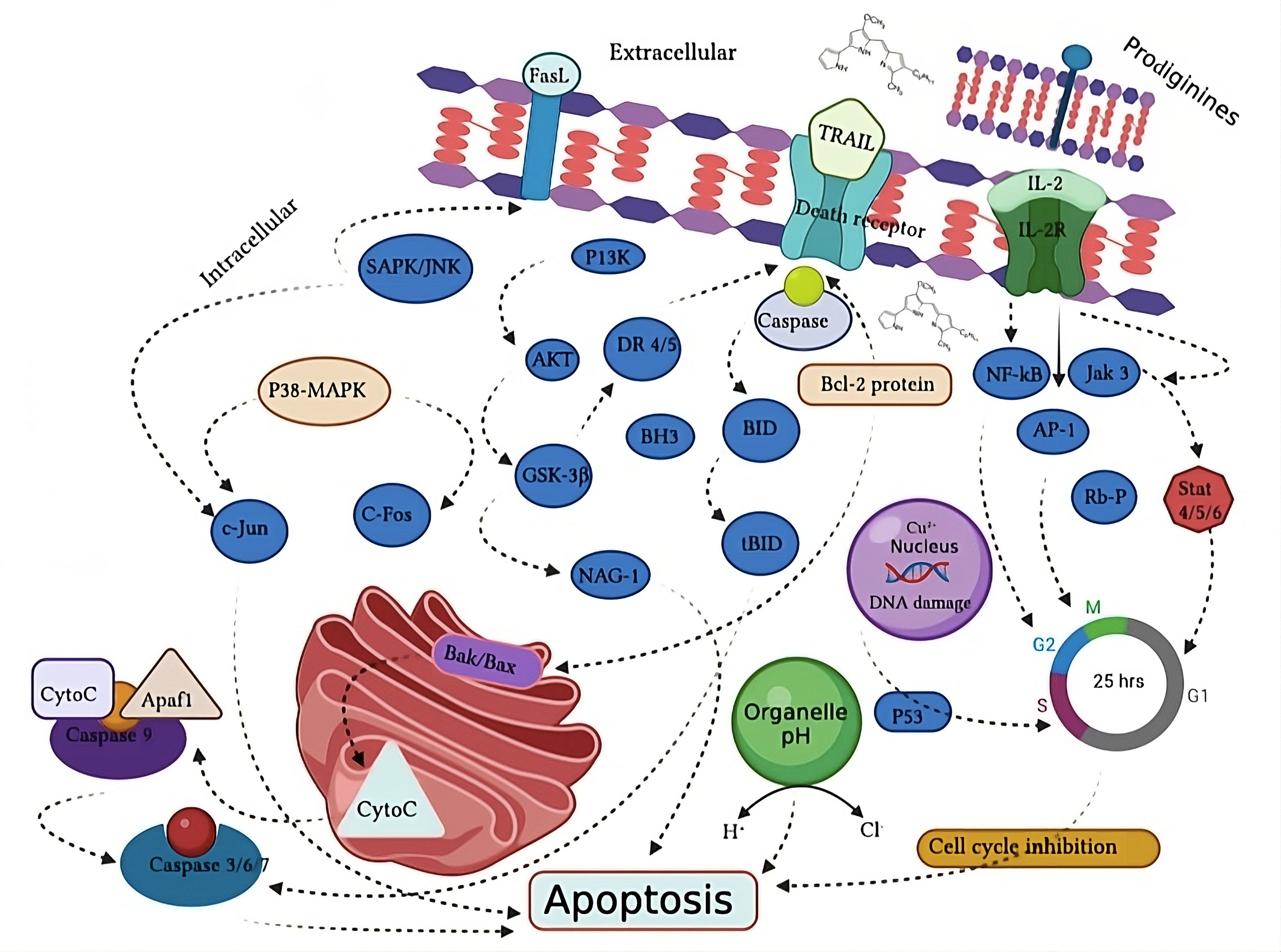 Figure 3. Prodigiosin prospective pathway of apoptosis induction in cancer cell. The prospective prodigiosin apoptosis induction pathway has been described in four different ways such as signal transduction, cell cycle blockage, intracellular acidification and DNA impairment. Figure designed by the adobe illustration tool.
Figure 3. Prodigiosin prospective pathway of apoptosis induction in cancer cell. The prospective prodigiosin apoptosis induction pathway has been described in four different ways such as signal transduction, cell cycle blockage, intracellular acidification and DNA impairment. Figure designed by the adobe illustration tool.
Further investigations on PG are required to confirm the compensatory mechanism for tumor inhibition and to know whether it may render B-cells proliferation or antitumorigenic activity. Additional information is needed to evaluate the provocative function of PG in inhibiting T-cell stimulation by curbing the expression of IL-2Rα in the IL-2/IL-2R signaling pathway. Limited research on prodigiosin encapsulation via nanoparticles reported that it might be an excellent alternative in tumor therapy, adding that this is an important area to focus on in future research to find fruitful results in cancer treatment. In conclusion, more deep analysis is needed to evaluate more functionalities in prodigiosin and the basis for its miscellaneous profiles regarding bioactivities in biomedical sciences.
Further investigations on PG are required to confirm the compensatory mechanism for tumor inhibition and to know whether it may render B-cells proliferation or antitumorigenic activity. Additional information is needed to evaluate the provocative function of PG in inhibiting T-cell stimulation by curbing the expression of IL-2Rα in the IL-2/IL-2R signaling pathway. Limited research on prodigiosin encapsulation via nanoparticles reported that it might be an excellent alternative in tumor therapy, adding that this is an important area to focus on in future research to find fruitful results in cancer treatment. In conclusion, more deep analysis is needed to evaluate more functionalities in prodigiosin and the basis for its miscellaneous profiles regarding bioactivities in biomedical sciences.
No applicable.
Ethics approval
No applicable.
Data availability
The data will be available upon request.
Funding
None.
Authors’ contribution
AS contributed to draft, critical revision of the article, figure production and submitted the final manuscript.
Competing interests
None.
- WHO. Global health estimates: leading causes of death, 2019. Retrieved from World Health Organization: https://www. who. int/data/gho, 2021.
- Deo S, Sharma J, Kumar S: GLOBOCAN 2020 report on global cancer burden: challenges and opportunities for surgical oncologists. Ann Surg Oncol 2022, 29(11): 6497-6500.
- Ruggeri B: Cancer 2021: new therapeutic approaches for the treatment of cancer building on advances in cancer biology and the molecular genetics of cancer. Curr Opin Pharmacol 2021, 60: 341-345.
- Gmeiner WH: Recent advances in our knowledge of mCRC tumor biology and genetics: A focus on targeted therapy development. Onco Targets Ther 2021, 14: 2121-2130.
- Gomez-Cadena A, Barreto A, Fiorentino S, Jandus C: Immune system activation by natural products and complex fractions: a network pharmacology approach in cancer treatment. Cell Stress 2020, 4(7): 154-166.
- Gangwar V, Garg A, Lomore K, Korla K, Bhat SS, Rao RP, Rafiq M, Kumawath R, Uddagiri BV et al: Immunomodulatory effects of a concoction of natural bioactive compounds-Mechanistic insights. Biomedicines 2021, 9(11): 1522.
- Pan P, Huang YW, Oshima K, Yearsley M, Zhang J, Arnold M, Yu J, Wang LS: The immunomodulatory potential of natural compounds in tumor-bearing mice and humans. Crit Rev Food Sci Nutr 2019, 59(6): 992-1007.
- Di Sotto A, Vitalone A, Di Giacomo S: Plant-derived nutraceuticals and immune system modulation: an evidence-based overview. Vaccines 2020, 8(3): 468.
- Pan P, Kang S, Wang Y, Liu K, Oshima K, Huang YW, Zhang J, Yearsley M, Yu J, Wang LS et al: Black raspberries enhance natural killer cell infiltration into the colon and suppress the progression of colorectal cancer. Front Immunol 2017, 8: 997.
- Furugaki K, Pokorna K, Le Pogam C, Aoki M, Reboul M, Bajzik V, Krief P, Janin A, Noguera ME, West R et al: DNA vaccination with all-trans retinoic acid treatment induces long-term survival and elicits specific immune responses requiring CD4+ and CD8+ T-cell activation in an acute promyelocytic leukemia mouse model. J Am Society Hematol 2010, 115(3): 653-656.
- Huang X, Pan J, Xu F, Shao B, Wang Y, Guo X, Zhou S: Bacteria‐based cancer immunotherapy. Adv Sci 2021, 8(7): 2003572.
- Yip CH, Mahalingam S, Wan KL, Nathan S: Prodigiosin inhibits bacterial growth and virulence factors as a potential physiological response to interspecies competition. PLoS One 2021, 16(6): e0253445.
- Jansson J, Nilsson J, Modig F, Hed Vall G: Commitment to sustainability in small and medium‐sized enterprises: The influence of strategic orientations and management values. Bus Strategy Environ 2017, 26: 69-83.
- Suryawanshi RK, Patil CD, Koli SH, Hallsworth JE, Patil SV: Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat Prod Res 2017, 31(5): 572-577.
- Berning L, Schlütermann D, Friedrich A, Berleth N, Sun Y, Wu W, Mendiburo MJ, Deitersen J, Brass HU, Skowron MA et al: Prodigiosin sensitizes sensitive and resistant urothelial carcinoma cells to cisplatin treatment. Molecules 2021, 26(5): 1294.
- Han SB, Lee CW, Yoon YD, Kang JS, Lee KH, Yoon WK, Kim YK, Lee K, Park SK, Kim HM et al: Effective prevention of lethal acute graft-versus-host disease by combined immunosuppressive therapy with prodigiosin and cyclosporine A. Biochem Pharmacol 2005, 70(10): 1518-1526.
- Suryawanshi RK, Koujah L, Patil CD, Ames JM, Agelidis A, Yadavalli T, Patil SV, Shukla D: Bacterial pigment prodigiosin demonstrates a unique antiherpesvirus activity that is mediated through inhibition of prosurvival signal transducers. J. Virol 2020, 94(13): e00251-20.
- Metcalfe S, Ashley N, Chen Z, Calne RY: Prodigiosin 25C: effect in vitro models for T cell activation and T cell cycling and in vivo for rat heart allografts. Int Arch Allergy Immunol 1993, 101(2): 132-135.
- Kurz CL, Chauvet S, Andrès E, Aurouze M, Vallet I, Michel GP, Uh M, Celli J, Filloux A, De Bentzmann S et al: Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. EMBO J 2003, 22(7): 1451-1460.
- Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP: Anticancer and immunosuppressive properties of bacterial prodiginines. Futur Med 2007, 2(6): 605-618.
- Wang ME, Kirken RA, Behbod F, Erwin-Cohen R, Stepkowski SM, Kahan BD: Inhibition of jak3 tyrosine kinase by pnu156804 blocks rat heart allograft rejection. Abstract# 1093. Transplant Proc 2000, 33(1-2): 201.
- Schloss PD, Allen HK, Klimowicz AK, Mlot C, Gross JA, Savengsuksa S, McEllin J, Clardy J, Ruess RW, Handelsman J et al: Psychrotrophic strain of Janthinobacterium lividum from a cold Alaskan soil produces prodigiosin. DNA Cell Biol 2010, 29(9): 533-541.
- Chia-Che C, Wei-Chuan C, Tsing-Fen H, Ho-Shing W, Yu-Hong W: Development of natural anti-tumor drugs by microorganisms. J Biosci Bioeng 2012, 115(5): 501-511.
- Stankovic N, Radulovic V, Petkovic M, Vuckovic I, Jadranin M, Vasiljevic B, Nikodinovic-Runic J: Streptomyces sp. JS520 produces exceptionally high quantities of undecylprodigiosin with antibacterial, antioxidative, and UV-protective properties. Appl Microbiol Biotechnol 2012, 96(5): 1217-1231.
- Kalivoda EJ, Stella NA, Aston MA, Fender JE, Thompson PP, Kowalski RP, Shanks RM: Cyclic AMP negatively regulates prodigiosin production by Serratia marcescens. Res Microbiol 2010, 161(2): 158-167.
- Su WT, Tsou TY, Liu HL: Response surface optimization of microbial prodigiosin production from Serratia marcescens. J Taiwan Inst Chem Eng 2011, 42: 217-222.
- Mahlen SD: Serratia infections: from military experiments to current practice. Clin Microbiol Rev 2011, 24(4): 755-791.
- Mo S, Kim Jh, Oh CH: Different effects of acidic pH shock on the prodiginine production in Streptomyces coelicolor M511 and SJM1 mutants. J Microbiol Biotechnol 2013, 23(10): 1454-1459.
- Harris AK, Williamson NR, Slater H, Cox A, Abbasi S, Foulds I, Simonsen HT, Leeper FJ, Salmond GP et al: The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species-and strain-dependent genome context variation. Microbioliology 2004, 150(11): 3547-3560.
- Venil CK, Velmurugan P, Lakshmanaperumalsamy P: Genomic environment of cueR and copA genes for prodigiosin biosynthesis by Serratia marcescens SB08. Romanian Biotechnol Lett 2009, 14(6): 4812-4819.
- Salamov VSA: Solovyevand A: Automatic annotation of microbial genomes and metagenomic sequences. Metagenomics and its applications in agriculture, biomedicine and environmental studies. 2011, p61-78.
- Fürstner A: Chemistry and biology of roseophilin and the prodigiosin alkaloids: a survey of the last 2500 years. Angew Chem Int Ed Engl 2003, 42(31): 3582-3603.
- Williamson NR, Simonsen HT, Ahmed RA, Goldet G, Slater H, Woodley L, Leeper FJ, Salmond GP: Biosynthesis of the red antibiotic, prodigiosin, in Serratia: identification of a novel 2‐methyl‐3‐n‐amyl‐pyrrole (MAP) assembly pathway, definition of the terminal condensing enzyme, and implications for undecylprodigiosin biosynthesis in Streptomyces. Mol Microbiol 2005, 56(4): 971-989.
- Balasubramaniam B, Alexpandi R, Darjily DR: Exploration of the optimized parameters for bioactive prodigiosin mass production and its biomedical applications in vitro as well as in silico. Biocatal Agric Biotechnol 2019, 22: 101385.
- Yip CH, Yarkoni O, Ajioka J, Wan KL, Nathan S: Recent advancements in high-level synthesis of the promising clinical drug, prodigiosin. Appl Microbiol Biotechnol 2019, 103(4): 1667-1680.
- Briukhovetska D, Dörr J, Endres S, Libby P, Dinarello CA, Kobold S: Interleukins in cancer: from biology to therapy. Nat Rev Cancer 2021, 21(8): 481-499.
- Ye Z, Shi Y, Lees-Miller SP, Tainer JA: Function and molecular mechanism of the DNA damage response in immunity and cancer immunotherapy. Front Immunol 2021, 12: 797880.
- Hiam-Galvez KJ, Allen BM, Spitzer MH: Systemic immunity in cancer. Nat Rev Cancer 2021, 21(6): 345-359.
- Darshan N, Manonmani H: Prodigiosin and its potential applications. J Food Sci Technol 2015, 52(9): 5393-5407.
- Pérez-Tomás R, Montaner B, Llagostera E, Soto-Cerrato V: The prodigiosins, proapoptotic drugs with anticancer properties. Biochem Pharmacol 2003, 66(8): 1447-1452.
- Soto-Cerrato V, Llagostera E, Montaner B, Scheffer GL, Perez-Tomas R: Mitochondria-mediated apoptosis operating irrespective of multidrug resistance in breast cancer cells by the anticancer agent prodigiosin. Biochem Pharmacol 2004, 68(7): 1345-1352.
- Francisco R, Pérez-Tomás R, Gimènez-Bonafé P, Soto-Cerrato V, Giménez-Xavier P, Ambrosio S: Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol 2007, 572(2-3): 111-119.
- Llagostera E, Soto‐cerrato V, Montaner B, Pérez‐tomás R: Prodigiosin induces apoptosis by acting on mitochondria in human lung cancer cells. Ann N Y Acad Sci 2003, 1010: 178-181.
- Park G, Tomlinson JT, Melvin MS, Wright MW, Day CS, Manderville RA: Zinc and copper complexes of prodigiosin: implications for copper-mediated double-strand DNA cleavage. Org Lett 2003, 5(2): 113-116.
- Tomás RP, Ruir CD, Montaner B: Prodigiosin induces cell death and morphological changes indicative of apoptosis in gastric cancer cell line HGT-1. Histol Histopathol 2001, 16(2): 415-421.
- Chiu WJ, Lin SR, Chen YH, Tsai MJ, Leong MK, Weng CF: Prodigiosin-emerged PI3K/Beclin-1-independent pathway elicits autophagic cell death in doxorubicin-sensitive and-resistant lung cancer. J Clin Med 2018, 7(10): 321.
- Wang Z, Li B, Zhou L, Yu S, Su Z, Song J, Sun Q, Sha O, Wang X, Jiang W et al: Prodigiosin inhibits Wnt/β-catenin signaling and exerts anticancer activity in breast cancer cells. Proc Nat Acad Sci 2016, 113(46): 13150-13155.
- Elahian F, Moghimi B, Dinmohammadi F, Ghamghami M, Hamidi M, Mirzaei SA: The anticancer agent prodigiosin is not a multidrug resistance protein substrate. DNA Cell Biol 2013, 32(3): 90-97.
- Nguyen VB, Chen SP, Nguyen TH, Nguyen MT, Tran TTT, Doan CT, Tran TN, Nguyen A D, Kuo YH, Wang SL et al: Novel efficient bioprocessing of marine chitins into active anticancer prodigiosin. Mar Drugs 2019, 18(1): 15.
- Li D, Liu J, Wang X, Kong D, Du W, Li H, Hse CY, Shupe T, Zhou D, Zhao K et al: Biological potential and mechanism of prodigiosin from Serratia marcescens subsp. lawsoniana in human choriocarcinoma and prostate cancer cell lines. Int J Mol Sci 2018, 19(11): 3465.
- Llagostera E, Soto-Cerrato V, Joshi R, Montaner B, Gimenez-Bonafé P, Pérez-Tomás R: High cytotoxic sensitivity of the human small cell lung doxorubicin-resistant carcinoma (GLC4/ADR) cell line to prodigiosin through apoptosis activation. Anticancer Drugs 2005, 16(4): 393-399.
- Anwar MM, Shalaby M, Embaby AM, Saeed H, Agwa MM, Hussein A: Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): preclinical insights. Sci Rep 2020, 10(1): 14706.
- Islan GA, Rodenak-Kladniew B, Noacco N, Duran N, Castro GR: Prodigiosin: A promising biomolecule with many potential biomedical applications. Bioengineered 2022, 13(6): 14227-14258.
- Jeong Y, Kim HJ, Kim S, Park SY, Kim H, Jeong S, Lee SJ: Enhanced large-scale production of Hahella chejuensis-derived prodigiosin and evaluation of its bioactivity. J Microbiol Biotechnol 2021, 31(12): 1624-1631.
- Luong VT, Le Thanh NS: Prodigiosin purification from Serratia marcescens M10 and its antitumor activities. Vietnam J Biotechnol 2021, 19: 289-299.
- Waldman AD, Fritz JM, Lenardo MJ: A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020, 20(11): 651-668.
- Wherry EJ, Kurachi M: Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 2015, 15(8): 486-499.
- Zhang J, He T, Xue L, Guo H: Senescent T cells: a potential biomarker and target for cancer therapy. EBio Medicine 2021, 68: 103409.
- Han SB, Kim HM, Kim YH, Lee CW, Jang ES, Son KH, Kim SU, Kim YK: T-cell specific immunosuppression by prodigiosin isolated from Serratia marcescens. Int J immunopharmacol 1998, 20(1-3): 1-13.
- Han SB, Park SH, Jeon YJ, Kim YK, Kim HM, Yang KH: Prodigiosin blocks T cell activation by inhibiting interleukin-2Rα expression and delays progression of autoimmune diabetes and collagen-induced arthritis. J Pharmacol Experimen Ther 2001, 299(2): 415-425.
- Lee MH, Kataoka T, Magae J, Nagai K: Prodigiosin 25-C suppression of cytotoxic T cells in vitro and in vivo similar to that of concanamycin B, a specific inhibitor of vacuolar type H+-ATPase. Biosci Biotechnol Biochem 1995, 59(8): 1417-1421.
- Das S, Ariizumi K, Cruz Jr PD: T-cell inhibitors: a bench-to-bedside review. Dermatitis 2012, 23(5): 195-202.
- Songia S, Mortellaro A, Taverna S, Fornasiero C, Scheiber EA, Erba E, Colotta F, Mantovani A, Isetta AM, Golay J et al: Characterization of the new immunosuppressive drug undecylprodigiosin in human lymphocytes: retinoblastoma protein, cyclin-dependent kinase-2, and cyclin-dependent kinase-4 as molecular targets. J Immunol 1997, 158(8): 3987-3995.
- Coronella-Wood JA, Hersh EM: Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother 2003, 52(12): 715-738.
- Fridman WH, Petitprez F, Meylan M, Chen TW, Sun CM, Roumenina LT, Sautès-Fridman C: B cells and cancer: To B or not to B? J Experiment Med 2020, 218(1): e20200851.
- Mortellaro A, Songia S, Gnocchi P, Ferrari M, Fornasiero C, D’Alessio R, Isetta A, Colotta F, Golay J: New immunosuppressive drug PNU156804 blocks IL-2-dependent proliferation and NF-κB and AP-1 activation. J Immunol 1999, 162(12): 7102-7109.
- Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P: Interferon‐γ reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor‐associated macrophages. Int J Cancer 2009, 125(2): 367-373.
- Pollard JW: Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer 2004, 4(1): 71-78.
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M: The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004, 25(12): 677-686.
- Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Lu X et al: VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 2011, 20(6): 701-714.
- Steenbrugge J, Breyne K, Demeyere K, De Wever O, Sanders NN, Van Den Broeck W, Colpaert C, Vermeulen P, Van Laere S, Meyer E et al: Anti-inflammatory signaling by mammary tumor cells mediates prometastatic macrophage polarization in an innovative intraductal mouse model for triple-negative breast cancer. J Exp Clin Cancer Res 2018, 37(1): 191.
- Giraudo E, Inoue M, Hanahan D: An amino-bisphosphonate targets MMP-9–expressing macrophages and angiogenesis to impair cervical carcinogenesis. J Clin Investig 2004, 114(5): 623-633.
- Ouyang W, O’Garra A: IL-10 family cytokines IL-10 and IL-22: from basic science to clinical translation. Immunity 2019, 50(4): 871-891.
- Nakashima T, Iwashita T, Fujita T, Sato E, Niwano Y, Kohno M, Kuwahara S, Harada N, Takeshita S, Oda T et al: A prodigiosin analogue inactivates NADPH oxidase in macrophage cells by inhibiting assembly of p47phox and Rac. J Biochem 2008, 143(1): 107-115.
- Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X: Tumor-associated macrophages: recent insights and therapies. Front Oncol 2020, 10: 188.
- Scarlett UK, Rutkowski MR, Rauwerdink AM, Fields J, Escovar-Fadul X, Baird J, Cubillos-Ruiz JR, Jacobs AC, Gonzalez JL, Weaver J et al: Ovarian cancer progression is controlled by phenotypic changes in dendritic cells. J Experiment Med 2012, 209(3): 495-506.
- Wang D, DuBois RN: Role of prostanoids in gastrointestinal cancer. J Clin Investig 2018, 128(7): 2732-2742.
- Lin Y, Xu J, Lan H: Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J hematol Oncol 2019, 12(1): 76.
- Caligiuri MA: Human natural killer cells. Blood J Am Society Hematol 2008, 112(3): 461-469.
- Fulton AM, Chong YC: Prostaglandin E2 receptor activity and susceptibility to natural killer cells. J leukoc Biol 1992, 51(2): 176-180.
- Kundu N, Ma X, Holt D, Goloubeva O, Ostrand-Rosenberg S, Fulton AM: Antagonism of the prostaglandin E receptor EP4 inhibits metastasis and enhances NK function. Breast Cancer Res Treat 2009, 117(2): 235-242.
- Harizi H: Reciprocal crosstalk between dendritic cells and natural killer cells under the effects of PGE2 in immunity and immunopathology. Cell Mol Immunol 2013, 10(3): 213-221.
- Pérez Tomás RE, Montaner Ramoneda B: Effects of the proapoptotic drug prodigiosin on cell cycle-related proteins in Jurkat T cells. Histol Histopathol 2003, 18(2): 379-385.
- Soto-Cerrato V, Vinals F, Lambert JR, Kelly JA, Perez-Tomas R: Prodigiosin induces the proapoptotic gene NAG-1 via glycogen synthase kinase-3β activity in human breast cancer cells. Mol Cancer Ther 2007, 6(1): 362-369.
- Davey NE, Travé G, Gibson TJ: How viruses hijack cell regulation. Trends Biochem Sci 2011, 36(3): 159-169.
- Suba K, Stalin A, Girija A, Raguraman R: Homology modelling and docking analysis of prodigiosin from Serratia marcescens. Biotechnology 2013, 55: 12897-12902.
- Zhou W, Zeng C, Liu R, Chen J, Li R, Wang X, Bai W, Liu X, Xiang T, Zhang L et al: Antiviral activity and specific modes of action of bacterial prodigiosin against Bombyx mori nucleopolyhedrovirus in vitro. Appl Microbiol Biotechnol 2016, 100(9): 3979-3988.
- Varghese FS, Rausalu K, Hakanen M, Saul S, Kümmerer BM, Susi P, Merits A, Ahola T: Obatoclax inhibits alphavirus membrane fusion by neutralizing the acidic environment of endocytic compartments. Antimicrob Agents Chemother 2017, 61(3): e02227-16.
- Varghese FS, van Woudenbergh E, Overheul GJ, Eleveld MJ, Kurver L, van Heerbeek N, van Laarhoven A, Miesen P, den Hartog G, de Jonge MI et al: Berberine and obatoclax inhibit SARS-Cov-2 replication in primary human nasal epithelial cells in vitro. Viruses 2021, 13(2): 282.
- Mao B, Le-Trilling VTK, Wang K, Mennerich D, Hu J, Zhao Z, Zheng J, Deng Y, Katschinski B, Xu S et al: Obatoclax inhibits SARS-CoV-2 entry by altered endosomal acidification and impaired cathepsin and furin activity in vitro. Emerg Microbes Infect 2022, 11(1): 483-497.
- Patil CD, Suryawanshi RK, Koujah L, Shukla D: Antiviral efficacy of prodigiosin against corneal herpes simplex virus infection. Investig Ophthalmol Vis Sci 2020, 61: 2982-2982.
- Montaner B, Pérez-Tomás R: Prodigiosin-induced apoptosis in human colon cancer cells. Life Sci 2001, 68(17): 2025-2036.
- Monge M, Vilaseca M, Soto-Cerrato V, Montaner B, Giralt E, Pérez-Tomás R: Proteomic analysis of prodigiosin-induced apoptosis in a breast cancer mitoxantrone-resistant (MCF-7 MR) cell line. Invest New Drugs 2007, 25(1): 21-29.
- Montaner B, Prez-Toms R: Prodigiosins induces caspase-9 and caspase-8 activation and cytochrome C release in jurkat T cells. Ann N Y Acad Sci 2002, 1: 246-249.
- Liu R, Cui CB, Duan L, Gu QQ, Zhu WM: Potent in Vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Sac-charopolyspora sp. nov. Arch. Pharm. Res 2005, 28(12): 1341-1344.
- Ho TF, Ma CJ, Lu CH, Tsai YT, Wei YH, Chang JS, Lai JK, Cheuh PJ, Yeh CT, Tang PC et al: Undecylprodigiosin selectively induces apoptosis in human breast carcinoma cells independent of p53. Toxicol Appl Pharmacol 2007, 225(3): 318-328.
- Yamamoto D, Kiyozuka Y, Uemura Y, Yamamoto C, Takemoto H, Hirata H, Tanaka K, Hioki K, Tsubura A: Cycloprodigiosin hydrochloride, a H+/Cl− symporter, induces apoptosis in human breast cancer cell lines. J Cancer Res Clin Oncol 2000, 126(4): 191-197.
- Yamamoto D, Uemura Y, Tanaka K, Nakai K, Yamamoto C, Takemoto H, Kamata K, Hirata H, Hioki K: Cycloprodigiosin hydrochloride, H+/CL–symporter, induces apoptosis and differentiation in HL‐60 cells. Intl J Cancer 2000, 88(1): 121-128.
- Zhang J, Shen Y, Liu J, Wei D: Antimetastatic effect of prodigiosin through inhibition of tumor invasion. Biochem Pharmacol 2005, 69(3): 407-414.
- Liu Y, Zhou H, Ma X, Lin C, Lu L, Liu D, Ma D, Gao X, Qian XY: Prodigiosin inhibits proliferation, migration, and invasion of nasopharyngeal cancer cells. Cell Physiol Biochem 2018, 48(4): 1556-1562.
- Lin PB, Shen J, Ou PY, Liu LY, Chen ZY, Chu FJ, Wang J, Jin XB: Prodigiosin isolated from Serratia marcescens in the Periplaneta americana gut and its apoptosis‑inducing activity in HeLa cells. Oncol Rep 2019, 41(6): 3377-3385.
- Castillo-Ávila W, Abal M, Robine S, Pérez-Tomás R: Non-apoptotic concentrations of prodigiosin (H+/Cl− symporter) inhibit the acidification of lysosomes and induce cell cycle blockage in colon cancer cells. Life Sci 2005, 78(2): 121-127.
- Lucena T, Arahal DR, Ruvira MA, Navarro-Torre S, Mesa J, Pajuelo E, Rodriguez-Llorente ID, Rodrigo-Torres L, Pinar MJ, Pujalte MJ et al: Vibrio palustris sp. nov. and Vibrio spartinae sp. nov., two novel members of the Gazogenes clade, isolated from salt-marsh plants (Arthrocnemum macrostachyum and Spartina maritima). Int J Syst Evol Microbiol 2017, 67(9): 3506-3512.
- Hassankhani R, Sam MR, Esmaeilou M, Ahangar P: Prodigiosin isolated from cell wall of Serratia marcescens alters expression of apoptosis-related genes and increases apoptosis in colorectal cancer cells. Med Oncol 2015, 32(1): 1-8.
- Yenkejeh R, Sam M, Esmaeillou M: Targeting survivin with prodigiosin isolated from cell wall of Serratia marcescens induces apoptosis in hepatocellular carcinoma cells. Hum Exp Toxicol 2017, 36(4): 402-411.
- Lins JCL, DE MELO MEB, Do Nascimento SC, Adam ML: Differential genomic damage in different tumor lines induced by prodigiosin. Anticancer Res 2015, 35(6): 3325-3332.
- Soliev AB, Hosokawa K, Enomoto K: Effects of prodigiosin family compounds from Pseudoalteromonas sp. 1020R on the activities of protein phosphatases and protein kinases. J Enzyme Inhib Med Chem 2015, 30(4): 533-538.
- Ramoneda BM, Pérez-Tomás R: Activation of protein kinase C for protection of cells against apoptosis induced by the immunosuppressor prodigiosin. Biochem Pharmacol 2002, 63(3): 463-469.
- Montaner B, Pérez-Tomás R: The cytotoxic prodigiosin induces phosphorylation of p38-MAPK but not of SAPK/JNK. Toxicol Lett 2002, 129(1-2): 93-98.
- Montaner B, PÉREZ‐TOMÁS R: Prodigiosin Induces Caspase‐9 and Caspase‐8 Activation and Cytochrome C Release in Jurkat T Cells. Ann N Y Acad Sci 2002, 973: 246-249.
- Lu CH, Lin SC, Yang SY, Pan MY, Lin YW, Hsu CY, Wei YH, Chang JS, Chang CC: Prodigiosin-induced cytotoxicity involves RAD51 down-regulation through the JNK and p38 MAPK pathways in human breast carcinoma cell lines. Toxicol Lett 2012, 212(1): 83-89.
- Cheng SY, Chen NF, Kuo HM, Yang SN, Sung CS, Sung PJ, Wen ZH, Chen WF: Prodigiosin stimulates endoplasmic reticulum stress and induces autophagic cell death in glioblastoma cells. Apoptosis 2018, 23(5-6): 314-328.
- Soto-Cerrato V, Viñals F, Lambert JR, Pérez-Tomás R: The anticancer agent prodigiosin induces p21WAF1/CIP1 expression via transforming growth factor-beta receptor pathway. Biochem Pharmacol 2007, 74(9): 1340-1349.
- Pan MY, Shen YC, Lu CH, Yang SY, Ho TF, Peng YT, Chang CC: Prodigiosin activates endoplasmic reticulum stress cell death pathway in human breast carcinoma cell lines. Toxicol Appl Pharmacol 2012, 265(3): 325-334.
- Hong B, Prabhu VV, Zhang S, van den Heuvel APJ, Dicker DT, Kopelovich L, El-Deiry W S: Prodigiosin Rescues Deficient p53 Signaling and Antitumor Effects via Upregulating p73 and Disrupting Its Interaction with Mutant p53Rescuing Deficient p53 via p73 in Cancers. Cancer Res 2014, 74(4): 1153-1165.
- Dalili D, Fouladdel S, Rastkari N, Samadi N, Ahmadkhaniha R, Ardavan A, Azizi E: Prodigiosin, the red pigment of Serratia marcescens, shows cytotoxic effects and apoptosis induction in HT-29 and T47D cancer cell lines. Nat Prod Res 2012, 26(22): 2078-2083.
- Cheng MF, Lin CS, Chen YH, Sung PJ, Lin SR, Tong YW, Weng CF: Inhibitory growth of oral squamous cell carcinoma cancer via bacterial prodigiosin. Mar Drugs 2017, 15(7): 224.
- Espona-Fiedler M, Soto-Cerrato V, Hosseini A, Lizcano JM, Guallar V, Quesada R, Gao T, Pérez-Tomás R: Identification of dual mTORC1 and mTORC2 inhibitors in melanoma cells: prodigiosin vs. obatoclax. Biochem Pharmacol 2012, 83(4): 489-496.
- Sajjad W, Ahmad S, Aziz I, Azam SS, Hasan F, Shah AA: Antiproliferative, antioxidant and binding mechanism analysis of prodigiosin from newly isolated radio-resistant Streptomyces sp. strain WMA-LM31. Mol Biol Rep 2018, 45(6): 1787-1798.
- Borić M, Danevčič T, Stopar D: Prodigiosin from Vibrio sp. DSM 14379; a new UV-protective pigment. Microb Ecol 2011, 62(3): 528-536.
- Song K, Li J, Yang F, Wu Z, Chen W, Li P, Ling F, Wang G: Antiviral effect of prodigiosin isolated from fish intestinal bacteria against Micropterus salmoides rhabdovirus. Aquaculture 2023: 739683.
- Ge M, Gong M, Jiao Y, Li Y, Shen L, Li B, Wang Y, Wang F, Zhang S, Yang J: Serratia marcescens‐S3 inhibits Potato virus Y by activating ubiquitination of molecular chaperone proteins NbHsc70‐2 in Nicotiana benthamiana. Microb Biotechnol 2022, 15(4): 1178-1188.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript