Research Article | Open Access
Evaluation and formulation of antiseptic herbal hand wipes
S.M. Stefi1, Jerrine Joseph2, Mary Shamya A2, V. Ramesh Kumar1, Arumugam Suresh3, Rajasekar Thirunavukarasu4
1Department of Biotechnology, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India.
2Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India.
3Central Research Laboratory Meenakshi Medical College Hospital & Research Institute, Chennai, Tamil Nadu, India.
4Meenakshi Academy of Higher Education and Research Institute, Raasi Nagar, Karrapettai Post, Enathur, Tamil Nadu, India.
Correspondence: Jerrine Joseph (Centre for Drug Discovery and Development, Sathyabama Institute of Science and Technology, Chennai 600119, Tamil Nadu, India; E-mail: jerrine.jj@gmail.com).
Asia-Pacific Journal of Pharmacotherapy & Toxicology 2022, 2: 11-17.https://doi.org/10.32948/ajpt.2022.12.30
Received: 27 Dec 2022 | Accepted: 30 Dec 2022 | Published online: 31 Dec 2022
Methods Antimicrobial (Agar well diffusion method), antioxidant (DPPH assay), toxicity studies (using Zebra fish embryo) were carried out in this study.
Results The 0.5-2µg/ml of essential oil showed promising antimicrobial activity against standard routine bacterial pathogens, and also for Mycobacterium smegmatis wild strains. 100µg/ml of Tea tree oil and cinnamon oil were toxic to zebrafish embryo. However, sub-lethal doses of oils is (100 μg/mL) against Zebrafish embryo.
Conclusion Thus, an eco-friendly, safe, low-cost herbal hand wipes are formulated for human use for prevention and protection against microbial infection and for maintaining personal hygiene.
Key words aloe vera, essential oils, herbal wet wipes, antioxidant, embryo toxicity
Chemicals and solvents used in this experiment are of analytical grade.
Collection and processing of Aloe vera
The plant Aloe Veras were collected from Chintadripet, Nimilicherry and Mandampakkam of Chennai, Tamil Nadu in November 2019 and the voucher specimen was identified and validated by a botanist. The freshly harvested leaves of aloe vera were cut manually for experimentation. To avoid biodegradation the leaves are pulled from the mother plant carefully not to break the rind and packed in an icebox at 4-5°C and transported to the laboratory. The leaves were washed completely with fresh water. The outer layer of aloe vera was cut using a knife and the gel inside was taken separately and placed in a beaker. After taking the full gel in the beaker it was homogenized by blender to form the viscous solution.
Formulation of herbal solution
To the Aloe vera solution with preservatives, essential oils were added. This was done in four combinations. In 65% prepared solution 0.5% of tea tree oil and 0.5% of cinnamon oil were mixed separately and added. Lavender oil, basil oil and lemon oil were added based on fragrance. Also, a guar gum of 0.05% was dissolved with 30ml water and added to the solution. 0.5% of Quercetin was mixed with 2ml of acetone or ethanol and this was also separately added in the solution. 0.5ml of DMSO dissolved in 1.5ml of distilled water and added to the solution to the makeup of 100ml.To the other three combinations, different concentrations of EO’s, gum, and quercetin with the stabilizing agent was added.
Addition of preservatives
Preservatives are added to promote the shelf life of the gel and its stability. Preservatives such as potassium sorbate of 0.1%, sodium benzoate of 0.1%, glycerine 0.1%, vitamin E 3mg/100ml, vitamin D 3mg/100ml, citric acid of 0.1% was added to the processed viscous solution of Aloe vera [9].
Antioxidant assay
DPPH radical scavenging activity. The free radical scavenging capacity of the plant extract was measured based on the method delineate by Brand-Williams et al [10]. with slight modification. It is based on electron-transfer that produces a violet solution in methanol. This free radical, which is stable at room temperature, is reduced to colorless methanol solution in the presence of an antioxidant molecule [11]. 0.1 mM DPPH solution was mixed with 1ml of plant extract solution of varying concentration (10, 100, 500, 1000μg/ml) tubes was incubated at room temperature for 30 minutes in dark. Then the absorbance was measured using a UV-Vis spectrophotometer at 517 nm. Gallic acid was used as the standard [12]. The capability of scavenging the DPPH radical was calculated by using the formula:
DPPH scavenging effect/activity
(%inhibition or %scavenging) = {(A0-A1)/A0)x100}
Preparation and evaluation of antimicrobial herbal formulation
(1) Agar Well diffusion method. The antimicrobial activity of formulated solutions was screened by well diffusion method. The test strains used are S. aureus ATCC 29213, E.coli ATCC 25922, P.aeroginosa ATCC 27853, E.faecalis ATCC 29212, C.crusei ATCC 6258, C.albicans ATCC, and Mycobacterium smegmatis culture were collected from Lingam Microbiological Laboratory. The plates prepared with 15ml of Muller Hinton agar, lawn culture were made in each plate with standard testing strains, by using well puncture, well was made 15µl of formulated solution were added to the well. Gentamicin was used as a positive control and water as a negative control. The plates were incubated at 37°C for 24 h. After 24 hours of incubation, the test determines the efficacy of the formulation in terms of the zone of inhibition of the organism. The higher the zone of inhibition, the test sample will be more effective [13].
(2) Minimum inhibitory concentration. The MIC is defined as the lowest concentration which completely inhibits the growth of microorganisms for 24 hrs incubation. Determination of minimum inhibitory concentration of formulation was determined by preparing different concentrations 200μg, 400μg, and 800μg were added respectively to the Muller Hinton broth. A 50μl volume of each dilution was added aseptically into the wells of Mueller Hinton agar plates that were already inoculated with test bacteria. The agar plates were incubated at 37°C for 24 hours. The lowest concentration of oils showing a clear zone of inhibition was considered as the MIC.
Zebra fish embryo toxicity studies
Zebra fish embryos were purchased from the zebrafish aquarium in the Kanchipuram district. For toxicity studies, 10 healthy posts hatched zebrafish were transferred to the wells of a 24-well plate along with 1ml of embryo water (60 mg of sea salt/liter of ultrapure water). Different concentrations of wet wipe formulation (5, 10, 25, 50 and100 μg ml-1) were added to the wells and incubated for 72hrs at 28.5°C [14]. Mortality of the zebrafish was noted after 24, 48 and 72 hrs. At the end of the incubation period, the embryos were photographed using the stereomicroscope.
Statistical analysis
All invitro assay data signify the mean ± standard deviation of triplicates and IC50 was calculated by using one way analysis of variances (ANOVA). The inhibition concentration for each microorganism was analyzed using one-way analysis of variance (ANOVA). P value < 0.05 was considered as significant.
The plant was collected from different places of Chennai and was processed at Centre for Drug and Discovery Development, Sathyabama Institute of Science and Technology and the classification is represented in Table 1. The plant was sliced and the gel was separated and placed in a beaker. The gel was ground and made into a viscous solution in Figure 1.
Antioxidant activity by DPPH assay
(1) Antioxidant activity of Aloe vera extract and essential oils. The free radical scavenging capacity of the plant extract was measured with DPPH (2,2-diphenyl-1-picrylhydrazyl) method. It is a rapid and simple method to measure the ability of essential oils and the crude extract of Aloe vera act as free radical scavengers. In this method the maximum level of crude extract of Aloe vera inhibition was found to be 50% at 1000μg/ml. This can be seen in Figure 2. And for the essential oil Lemon grass oil showed maximum level of inhibition of 48% at 1000 μg/ml) and Citronella showed minimum level of inhibition and the IC50 value was also determined in Figure 3.
(2) Formulation of herbal solution. The formulation was prepared in four combinations with different concentrations of EO’s, gum, quercetin were shown in Table 2.
Microbial assay
(1) Anti microbial assay of different formulation. The microbial activity of different formulation was performed against different pathogens (Staph aurues, E.coli, P.aeruginosa, K.pneumoniae and C.albicans after 24 hours of incubation the zone of inhibition was noted. Whereas M.smegmatis took 24-48 hours to show the zone of inhibition. Formulation one showed the highest zone of inhibition against Klebsiella pneumonia, formulation 2 and 3 showed the highest zone against Staphylococcus aureus represented in Figure 4.
(2) Minimum inhibitory concentration. This method is used to measure the lowest concentration of the sample which inhibits the growth of the specific pathogen. However formulation 1 showed the lowest inhibitory concentration (100 µg/ml) against C.albicans, where formulation 2 showed minimal inhibitory activity against C.albicans at (250µg/ml), S.aureus at (62.5µg/ml) and E.coli (500µg/ml) and formulation 3 showed lowest concentrations against S.aureus at (100µg/ml). According to statistical analysis results of one way ANOVA of Staphylococcus aureus the P-value was 0.306, Candida albicans; P-value was 0.808, Escherichia coli P-value was 0.530. However the P-values were greater than 0.05 were described in Table 3. As a result, we fail to reject the null hypothesis and conclude that there is no significant variation in the MIC at 95% CI for each of the test organisms.
Time kill assay
This assay is used to determine the bacetriacidal or bacteriostatic activity of the essential oils over time. Based on the above results formulation 2 and 3 were chosen to check the bactericidal activity against Escherichia coli, Staphylococcus aureus, P.aeruoginosa ATCC 27853, E.faecalis ATCC 29212 and C.albicans ATCC. The results from this study show the potential of EO’s (Essential oil) as a blend in the ratio of 1:1:2μg/ml concentration that is formulation 3 acts as a therapeutic option to reduce bacterial colonization and infections from clinically resistant pathogens in Figure 5.
Toxicity on embryo of zebra fish
Toxicity was seen in the embryos of zebra fish from 24 to 72 hours and every day the picture was taken in a microscope. At this period different stages of zebrafish and also the movement was recorded. The zygote, cleavage, blastula, gastrula and segmentation were seen. This was seen for different concentration for the formulation 3 such as 20 μl, 40 μl, 60 μl, 80 μl, 100 μl and 500 μl were no toxicity was observed. Even in higher concentration also after 72 hours the embryos able to survive in the herbal formulation solution in all concentration Figure 6 and live rate of zebra fish embryo.
Fabrication and packing of wipes
The non woven fabric material was cut into 10cm and autoclaved to make the wipes sterile. The sterile wipes are placed on a tray and the formulated solution is poured on the material and allowed to settle for 5-10min so that the wipes absorbs the solution. It is then folded and placed inside silver pack and it is sealed in Figure 7.
Trademark registration
The future scope of the study is to register for the trademark for the product. Protocol for trademark are Receiving International applications, Verification of International applications, Certification and transmission of International application, Receiving irregularities if any, from the wipe and responding to them, Communication as to ceasing of effect, Receiving international registrations designating India, Examination of international registrations designating India, Publication of the international registration and Opposition proceedings.
 Figure 1. Extraction and preparation of viscous solution.
Figure 1. Extraction and preparation of viscous solution.
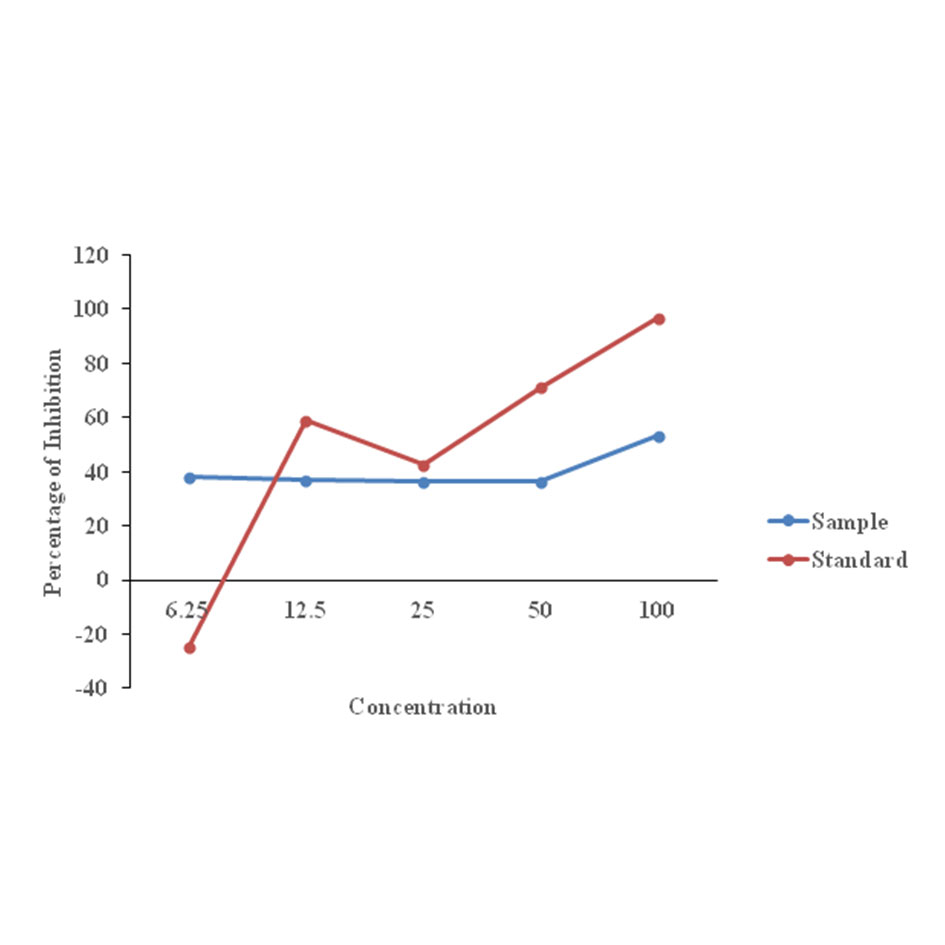 Figure 2. The antioxidant activity of the crude Aloe vera extract by DPPH method.
Figure 2. The antioxidant activity of the crude Aloe vera extract by DPPH method.
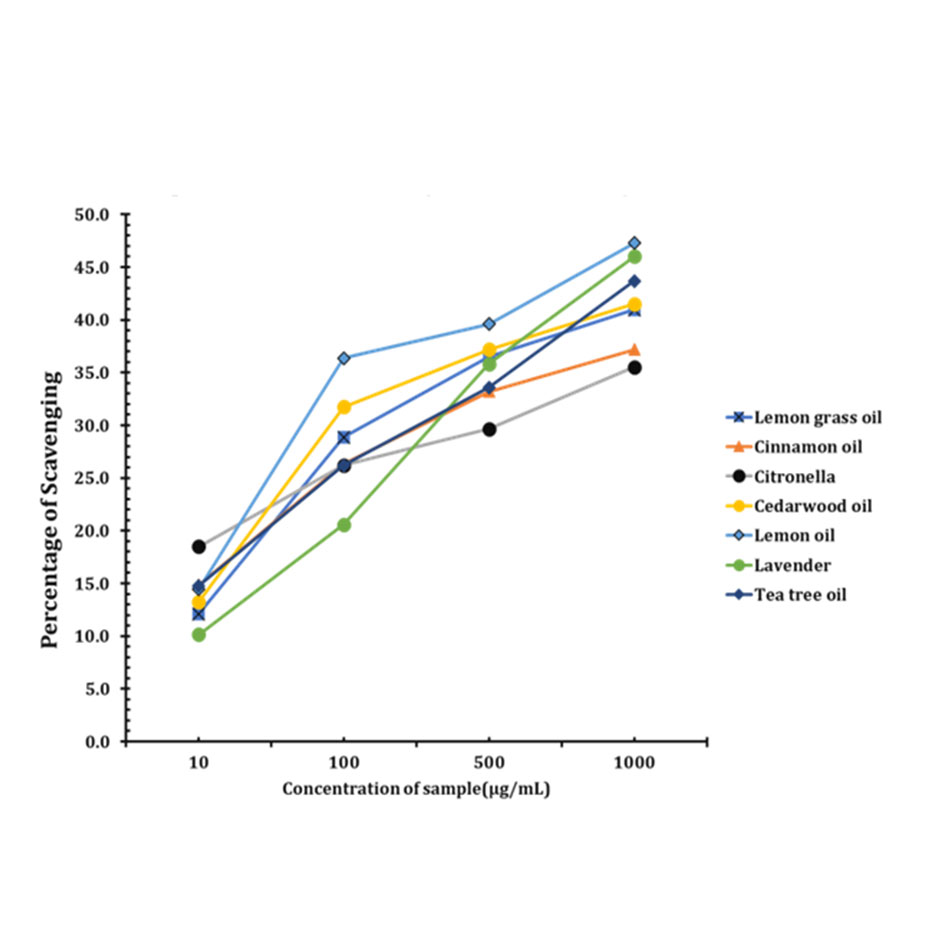 Figure 3. The antioxidant activity of the essential oils by DPPH method.
Figure 3. The antioxidant activity of the essential oils by DPPH method.
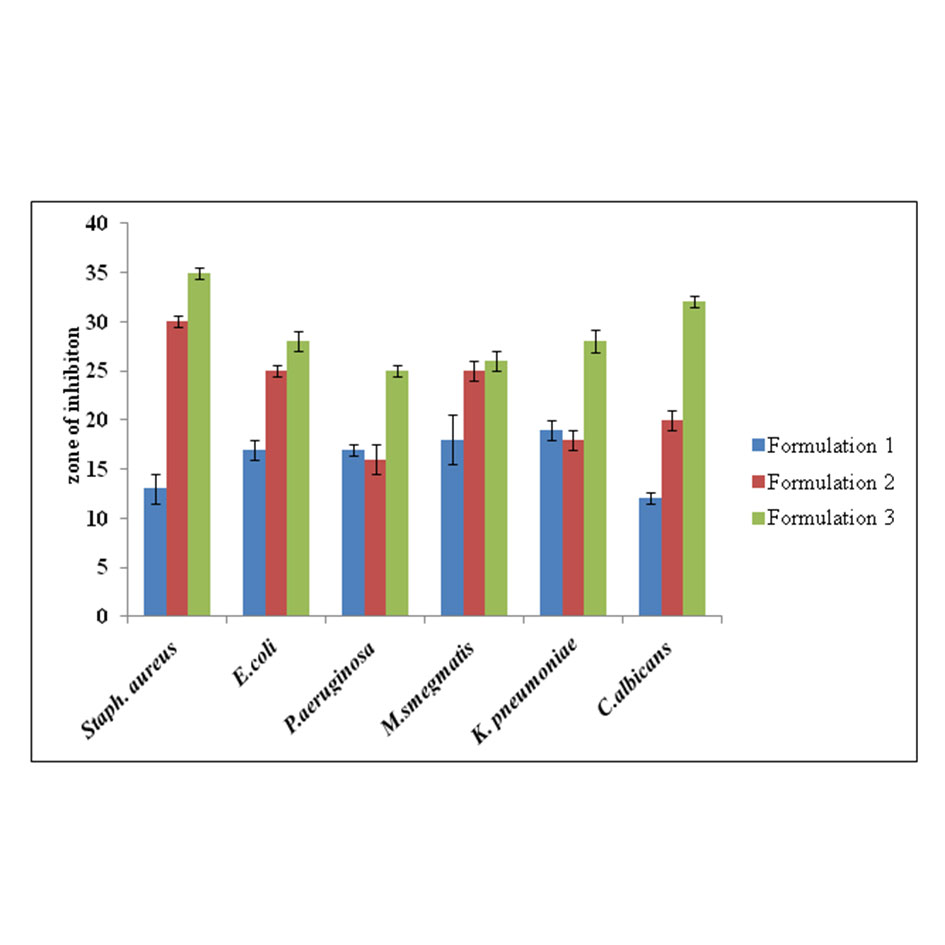 Figure 4. Antimicrobial assay of different formulations against various pathogens.
Figure 4. Antimicrobial assay of different formulations against various pathogens.
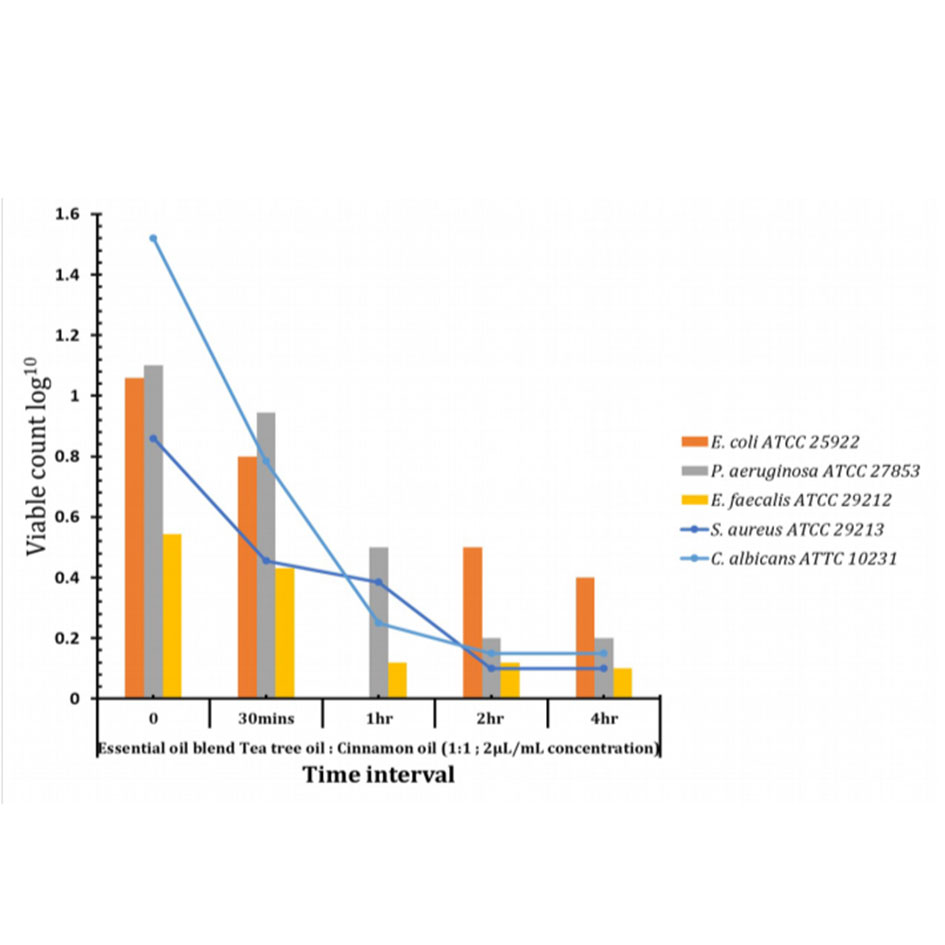 Figure 5. Time kill assay for essential oil blend.
Figure 5. Time kill assay for essential oil blend.
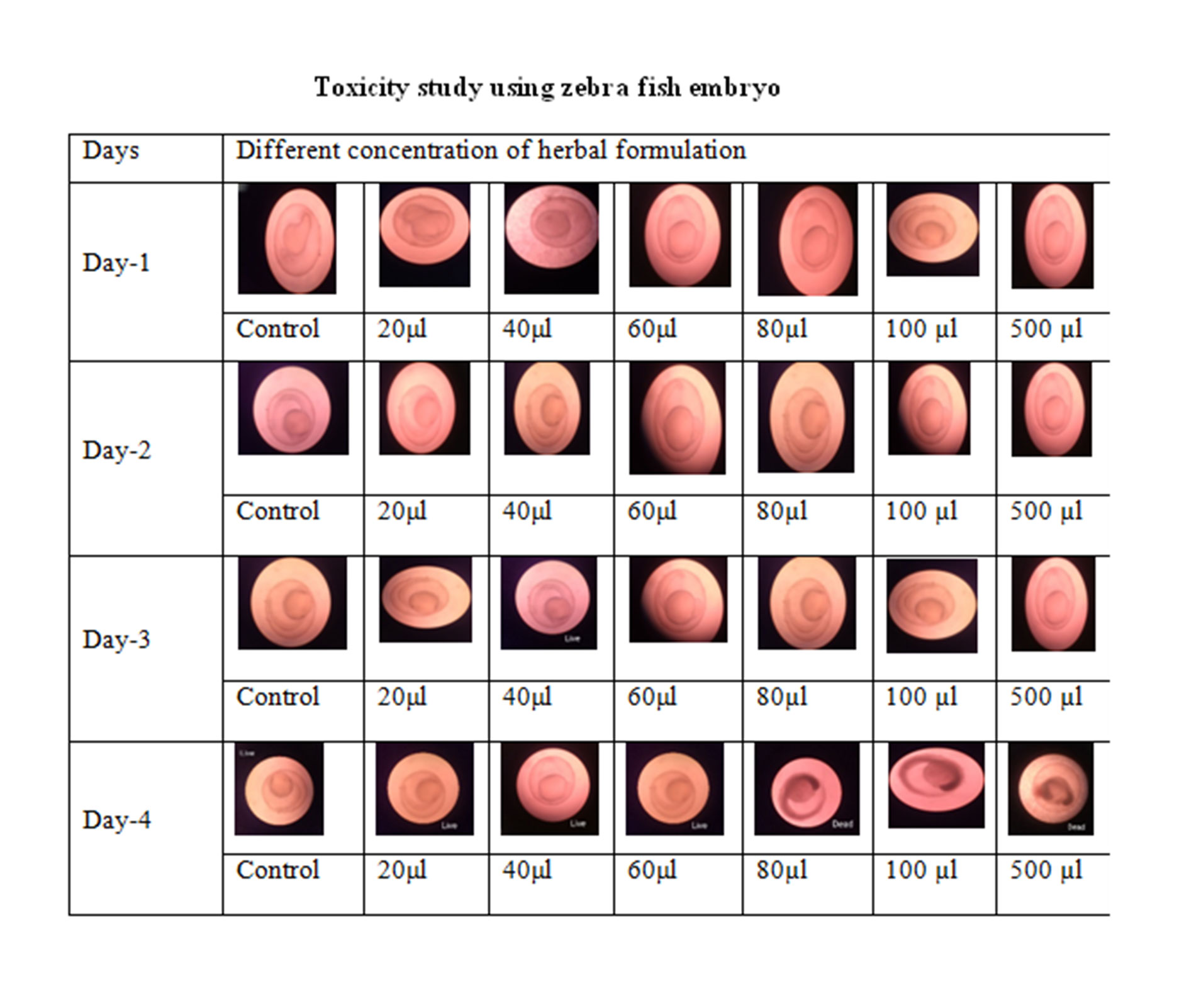 Figure 6. Fabrication and packing of herbal wipes.
Figure 6. Fabrication and packing of herbal wipes.
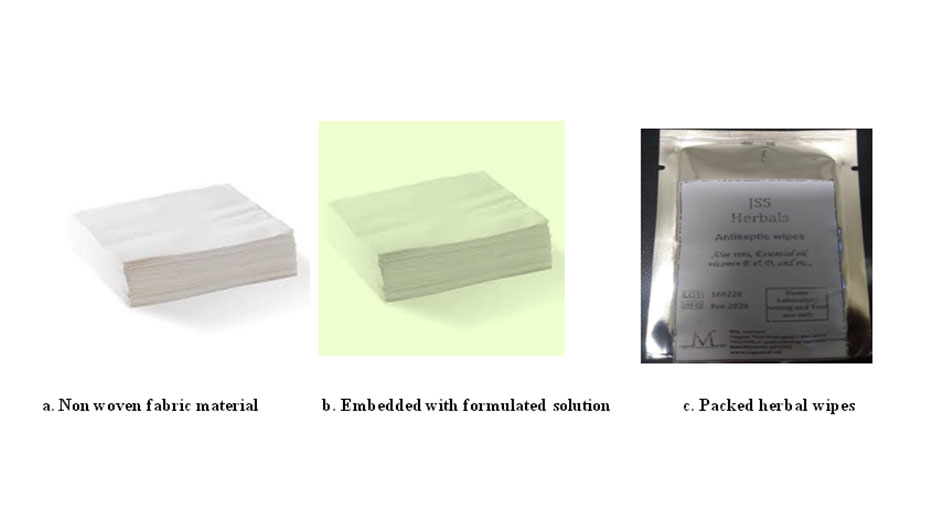 Figure 7. Zebrafish embryo toxicity of herbal formulation.
Figure 7. Zebrafish embryo toxicity of herbal formulation.
|
Table 1. Classification of Aloe vera. |
|
|
Kingdom |
Plantae |
|
Clade |
Tracheophytes |
|
Clade |
Angiosperms |
|
Clade |
Monocots |
|
Order |
Asparagales |
|
Family |
Asphodelaceae |
|
Subfamily |
Asphodeloideae |
|
Genus |
Aloe |
|
Species |
A. vera |
|
Table 2. Optimization of Essential oil concentration in antiseptic formulations. |
|||
|
Essential oil |
Formulation 1 |
Formulation 2 |
Formulation 3 |
|
Tea Tree oil |
1±0.1 |
0.3±0.3 |
0.01±0.2 |
|
Cinnamon oil |
1±0.2 |
0.3±0.1 |
0.01±0.1 |
|
Constant Base ratio |
Aloe vera with stabilizing agent-50v/v; Guar gum-0.1%; Vitamin E & D-0.01%; Quercetin-1% |
||
|
Table 3. Minimum inhibitory concentration of different herbal formulations against three different pathogens. |
|||||||||
|
Formulation 1 |
Formulation 2 |
Formulation 3 |
P value |
||||||
|
S.aureus
MIC µg/ml |
E. coli
MIC µg/ml |
C.albicans
MIC µg/ml |
S.aureus
MIC µg/ml |
E. coli
MIC µg/ml |
C.albicans
MIC µg/ml |
S.aureus
MIC µg/ml |
E. coli
MIC µg/ml |
C.albicans
MIC µg/ml |
|
|
- |
- |
- |
- |
500 |
- |
- |
- |
- |
0.05 |
|
- |
- |
- |
- |
- |
250 |
100 |
- |
- | |
|
- |
- |
100 |
- |
- |
- |
- |
- |
- | |
|
- |
- |
- |
62.5 |
- |
- |
- |
- |
- | |
The authors are grateful to the management of Sathyabama Institute of Science and Technology (deemed University) Chennai for the providing the infrastructure facility.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
Not applicable.
Author contributions
SMS carried out the entire lab work assays for the manuscript; JJ prepared draft document and invigilation for the manuscript; MSA carried out the extract preparation and antioxidant assay for the manuscript and VRK carried out correction and invigilation for the manuscript; AS carried out the trade marketing and antimicrobial study and RT carried out the grammer and correction for the manuscript.
Competing interests
All authors declare no competing interests.
- Siegert W: Microbiological quality management for the production of wet-wipes. Hous Person Car Tod 2008, 2.
- Acharya SB, Ghosh S, Yadav G, Sharma K, Ghosh S, Joshi S: Formulation, evaluation and antibacterial efficiency of water-based herbal hand sanitizer gel. BioRxiv 2018, 2018: 373928.
- Al-Zahrani SH, Baghdadi AM: Evaluation of the efficiency of Non alcoholic-Hand Gel Sanitizers products as an antibacterial. Nat & Sci 2012, 10(6): 15-20.
- Ahmad A, Husain A, Khan SA, Mujeeb M, Bhandari A: Synthesis, antimicrobial and antitubercular activities of some novel pyrazoline derivatives. J Saudi Chem Soc 2016, 20(5): 577-554.
- Christaki EV, Florou-Paneri PC: Aloe vera: a plant for many uses. J Food Agric Environ 2010, 8(2): 245-249.
- Maan A, Nazir A, Khan M, Ahmad T, Zia R, Murid M, Abrar M: The therapeutic properties and applications of Aloe vera: a review. J Herb Med 2018, 12: 1-10.
- Hamid AA, Aiyelaagbe OO, Usman LA: Essential oils: its medicinal and pharmacological uses. Int J Cur Res 2011, 33(2): 86-98.
- Swamy MK, Akhtar MS, Sinniah UR: Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid Based Complement Alternat Med 2016, 2016: 3012462.
- Nazemi N, Monfared A: Processing and stabilization of Aloe Vera leaf gel by adding chemical and natural preservatives. Res J Pharm 2017, Suppl(4): 24.
- Brand-Williams W, Cuvelier ME, Berset CL: Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Tech 1995, 28(1): 25-30.
- Miladi S, Damak M: In vitro antioxidant activities of Aloe vera leaf skin extracts. J Soc Chim Tunisie 2008, 10(10): 101-109.
- Garcia EJ, Oldoni TL, Alencar SM, Reis A, Loguercio AD, Grande RH: Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz Dent J 2012, 23(1): 22-27.
- Aref HL, Salah KB, Chaumont JP, Fekih A, Aouni M, Said K: In vitro antimicrobial activity of four Ficus carica latex fractions against resistant human pathogens (antimicrobial activity of Ficus carica latex). Pak J Pharm Sci 2010, 23(1): 53-58.
- Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, Bohannan BJ: The composition of the zebrafish intestinal microbial community varies across development. ISME J 2016, 10(3): 644-654.
- Pellizzoni M, Molinari GP, Lucini L: Stability of the main Aloe fractions and Aloe-based commercial products under different storage conditions. Agrochimica 2011, 55(5): 288-296.
- Bashir A, Saeed B, Mujahid TY, Jehan N: Comparative study of antimicrobial activities of Aloe vera extracts and antibiotics against isolates from skin infections. Afr J Biotech 2011, 10(19): 3835-3840.
- Sultana R, Begum R, Rahman MN, Hasan MR, Haque MA: Development of Aloe Vera Jelly for Diabetic Patients and Analysis of Its Physicochemical Properties. Int J Food Sci Biotech 2020, 5(1): 1-5.
- Ray A, Gupta SD, Ghosh S: Evaluation of anti-oxidative activity and UV absorption potential of the extracts of Aloe vera L. gel from different growth periods of plants. Indust Crops Prod 2013, 49: 712-719.
Asia-Pacific Journal of Pharmacotherapy & Toxicology
p-ISSN: 2788-6840
e-ISSN: 2788-6859
 Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.
Copyright © Asia Pac J Pharmacother Toxicol. This work is licensed under a Creative Commons Attribution-NonCommercial-No Derivatives 4.0 International (CC BY-NC-ND 4.0) License.

 Submit Manuscript
Submit Manuscript